1. How to read this document
One of the key principles of the GMP+ Feed Certification scheme is the chain approach: all links in the chain should take responsibility for assuring the safety of feed. Within GMP+ FSA certification module , this is arranged via purchasing requirements. In general, it is required to purchase feed and feed services from suppliers that fulfill the TS1.2 Purchase requirements.
This document is intended to guide the GMP+ certified companies through the purchasing requirements and also shows the scope of the purchase requirements. The examples are explained via a flowchart and a short description.
In chapter 2 the used symbols in the flowcharts are explained. Chapter 3 explains the different purchase options for feed, services and other products. Chapter 4 shows some situations per industry in text and flowcharts. Chapter 5 addresses questions received from the GMP+ Community.
This document is intended as a guidance to GMP+ FSA certification and should be considered as such. The requirements in the Feed Safety Management Requirements and Technical Specification (normative) documents of the GMP+ Feed Certification scheme must be followed.
This document gives a number of examples, but does not describe every possible situation. In the event of any doubt please contact GMP+ International.
All terms and definitions can be found in F0.2 Definition list.
2. Used figures & illustrations
Overview of figures & illustrations in this document:
| | A feed company is active in the feed supply chain. This can be either a producer or a trader. |
| | A food company is active in the food supply chain. |
| | A company active in the supply chain that is not a feed or a food company. |
| | The colors of this symbol indicate a food company that is producing feed as a by-product. |
| | The colors of the symbol indicate a company that is producing feed as a by-product. |
| | The by-product / feed material that is produced and supplied to a feed company. |
| | A livestock farmer. |
| | The GMP+ FSA logo in the flow chart indicates that the company / link in the supply chain must comply with the GMP+ FSA requirements and should be GMP+ FSA certified. |
| | Flow of feed products from one company to the other. |
| | Flow of non-feed products from one company to the other. |
| | Purchase using gatekeeper protocol requirements. |
| | Purchase using HACCP requirement. |
3. Purchasing requirements GMP+ FSA certification
In general products and services should be purchased from GMP+ FSA or equivalent certified suppliers. In some cases certification of the supplier is not mandatory and products and services can be purchased by using a gatekeeper protocol.
GMP+ International has various supporting products with information that can support in carrying out a hazard analysis: Feed Support Products (FSP). Its interactive nature and the information contained in it, offers a massive source of data which can be used in defining the risks of the feed ingredients purchased but also in drawing up the HACCP plans. The Feed Support Products can provide information about: the production process of feed materials, possible hazards, facts about hazards, the limits and monitoring data. Visit the GMP+ Portal to get to the FSP and search the feed ingredients purchased or produced, the flowchart in the risk assessment will give more detailed information.
3.1. Purchase from GMP+ certified suppliers
Purchase from GMP+ certified suppliers guarantees that all the links in the feed supply chain apply uniform and transparent safety assurance for products and services. Every supplier contributes to the safety of the products and services by applying the same GMP+ FSA basic principles (prerequisites, HACCP and system requirements).
3.2. Equivalent certification schemes
It is also accepted to purchase products and services from suppliers who are certified for an equivalent certification scheme. Even though there are differences between equivalent certification schemes, these differences are considered minor and result in the same level of feed safety assurance.
All equivalent certification schemes that are accepted by GMP+ International are stated in TS1.2 Purchase.
3.3. Gatekeeper options
For some specific situations, there are gatekeeper options available. Gatekeeper options are created because for some products and/or services it is not possible to demand GMP+ (or equivalent) certification. The conditions and possibilities for these gatekeeper options are described in TS1.2 Purchase.
A definition of gatekeeper can be found in F0.2 List of definitions. The gatekeeper needs to comply with specific requirements laid down in gatekeeper protocols. In these gatekeeper protocols, specific requirements are laid down to ensure feed safety assurance in previous links in the supply chain.
This includes, for example, a HACCP study, monitoring requirements and in some situations a supplier audit. This way the products or service can be brought into the GMP+ supply chain even though the supplier is not GMP+ certified.
Gatekeeper protocols are only available for a limited list of products and services. If there is no gatekeeper protocol available for (a group of) products or service, this means that the GMP+ certified company is not allowed to act as a gatekeeper and can only buy these products or service from a GMP+ (or equivalent) certified suppliers.
3.4. Purchase of other products and services via HACCP approach
Not every product or service purchased by a company needs to be covered under the scope of GMP+ FSA certification. These products or services can be purchased from uncertified suppliers, instead HACCP requirements are applicable. In addition to the HACCP requirements, the supplier and buyer should, as always, follow legal requirements regarding registration of producer and product and usage of the product.
Examples of ‘other’ products and services that can be purchased via a HACCP approach are:
- Cleaning agents
- Insecticides/fumigation
- Processing aids (not to be confused with technological feed additives)
- Veterinary medical products (feed medicines)*
- Raw materialsfor the production of feed additives and feed materials (See chapter 4 for the individual industries)
- Silo cleaning
- Pest control
- Etc.
The principles of HACCP are described in S9.4 Applying HACCP assessment.
| HACCP approach for suppliers of the first link in the GMP+ supply chain Suppliers of raw material do not themselves have to be GMP+ certified as they are not supplying feed. The GMP+ certified company should carry out a hazard analysis based on HACCP principles to ensure the production of safe feed. The hazard analysis should include all the activities, actions and/or processes related to the production of the raw material. On the basis of the result of this hazard analysis, on HACCP principles, and also on the quality assurance which is applied by the supplier, the GMP+ certified company will make a selection of suppliers and will adjust his (entry) check accordingly. More information about carrying out a hazard analysis can be found in S9.4 Applying HACCP assessment. |
*Veterinary medical products may be purchased from non-certified suppliers, under condition that these products:
- are registered or authorized by the competent authority;
- are purchased only on veterinary prescription (unless veterinary prescription is now obliged by law);
- when administered to food-producing animals, have a (provisional) MRL (maximum residue limit) set for the active substances, or if it has been determined that this limit is not necessary.
3.5. The start of GMP+ certification supply chain
The previous paragraphs explained the purchase options for GMP+ certified companies in text. Flowchart 1 will give a visible example of the purchase options. The next chapter will give some examples of the start of GMP+ certification supply chain and what purchase requirements needs to be followed.
Naturally, there should be compliance with the other requirements within the framework of supplier and product selection. Products should be permitted for the intended use by the relevant (national) authorities.
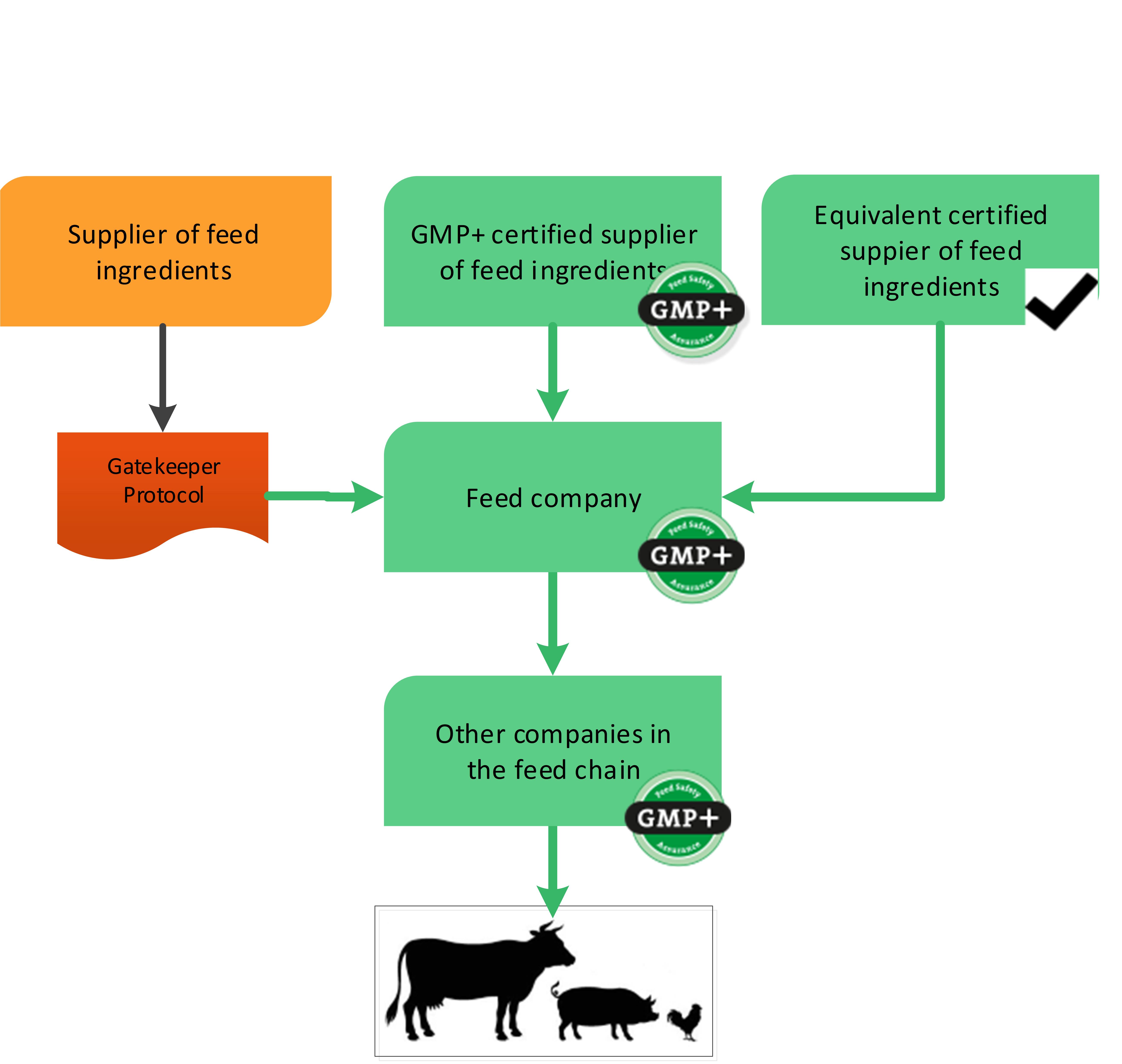
Flowchart 1: General purchase requirements
4. Specific feed supply chains
GMP+ purchase requirements are applicable from the link where the feed is produced, which is intended (or will be intended) for use in the feed sector: for example a feed material. The status of the product should be made clear by the producing company. In other words; if a feed is produced by a company, this is the starting point of GMP+ certification. The producer of the feed and each sub-sequent link in the GMP+ supply chain (including the traders and service providers) should comply with the GMP+ FSA requirements and should be certified accordingly.
4.1. The food sector
Food producers can deliver various types of products to the feed sector. Most common flows from food producers to the feed industry are “by-products” that are derived from the production process of food. That is the point where a feed material is created and the feed supply chain starts. And that is also the first link in the supply chain where GMP+ FSA certification is applicable. The definition of by-products, can be found in F 0.2.
Besides the production of by-products by food producing companies, there are other products that can be derived from food companies. These are foodstuff and former foodstuff or (food) additives. The definition of these products can be found in F 0.2 Definition list. Companies producing foodstuff and former foodstuff do not have to be GMP+ certified for the production of those as they were intended for food. A feed company can purchase these foodstuff or former foodstuff via a gatekeeper protocol. The purchase requirements can be found in TS 1.2 Purchase, paragraph 4.3.3 and 4.3.4.
First link in the GMP+ supply chain
The first link in the GMP+ supply chain is the point where feed is produced. The first link in the GMP+ supply chain is a food producing company when this food producing company is producing a by-product that is sold to the feed supply chain as a feed material.
The scope of the GMP+ feed safety management system starts at the moment of creation of the by-product / feed material up to and including sale to a feed company.
In case of production of a by-product, a food producing company should be aware that in addition to foodstuff, feed is produced as well. It is important to realize that the risks related to feed production can be different to the risks related to food production. Undesirable substances can, for example, be concentrated in the by-products.
For all flowcharts in paragraph 4.1 the scope of the GMP+ feed safety management system should include the production of the by-product up to and including its sale to the feed company.
Examples of food production supply chains
The following paragraphs (§ 4.1.1 until § 4.3) give an insight in some examples of food production supply chains where by-products are produced or where foodstuff or former foodstuff are delivered to the feed industry.
Note: The flowcharts in these paragraphs are intended as example to inform about the start of GMP+ certification. Of course not all supply chains are alike, nor do they have the same feed delivered to the feed supply chain. Use the examples in this document for the general explanation. In case of questions, contact GMP+ International for more information.
4.1.1. The sugar industry
A sugar producing factory is a foodstuff producing company (sugar) with a by-product which is used in the feed sector (for example sugar beet pulp). GMP+ certification begins at the sugar factory where the feed material sugar beet pulp is produced, see flowchart 2. In addition to this, also the sugar can be sold to feed companies, classified as a foodstuff. See flowchart 3 for an example.
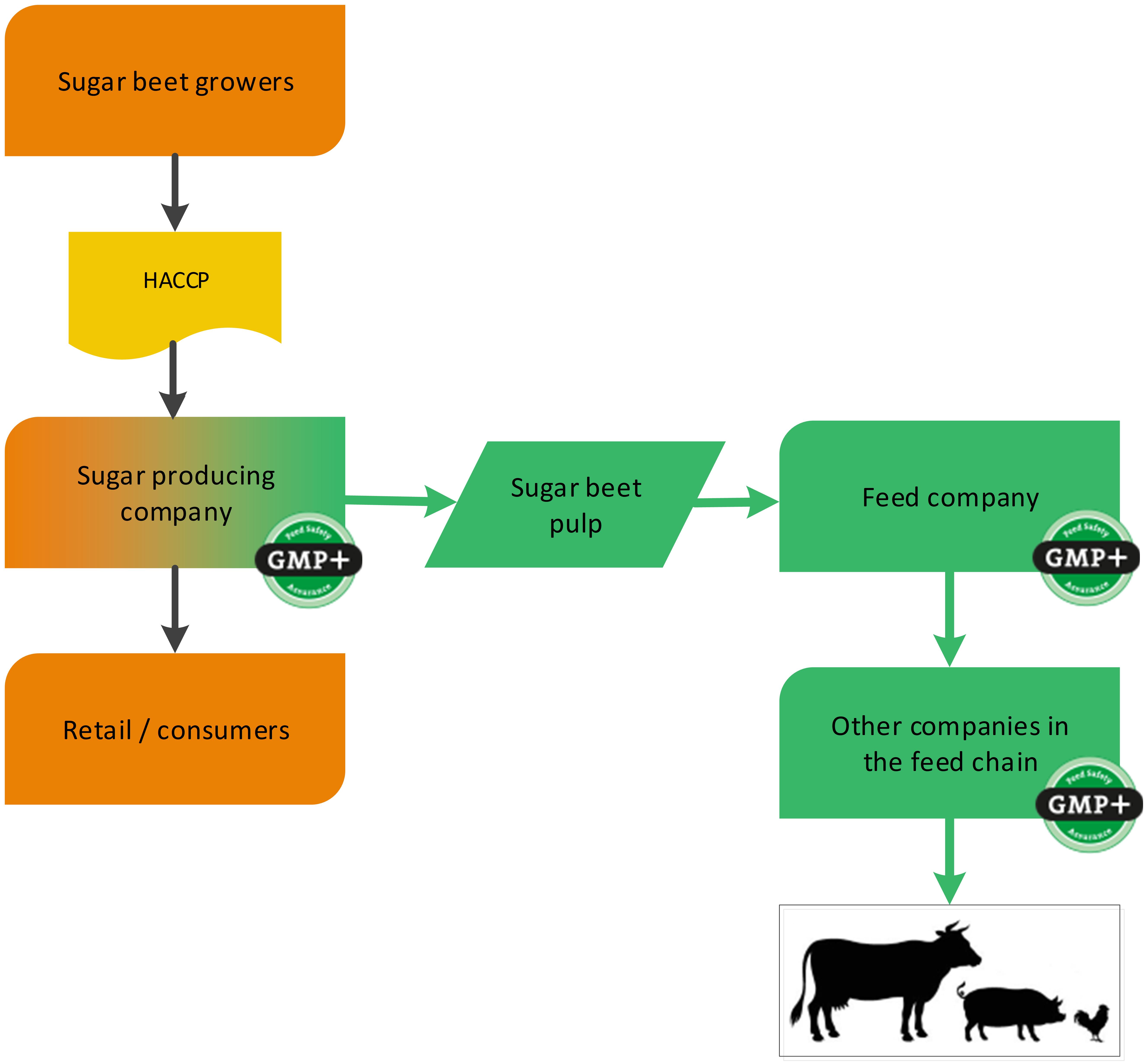
Flowchart 2: The sugar industry
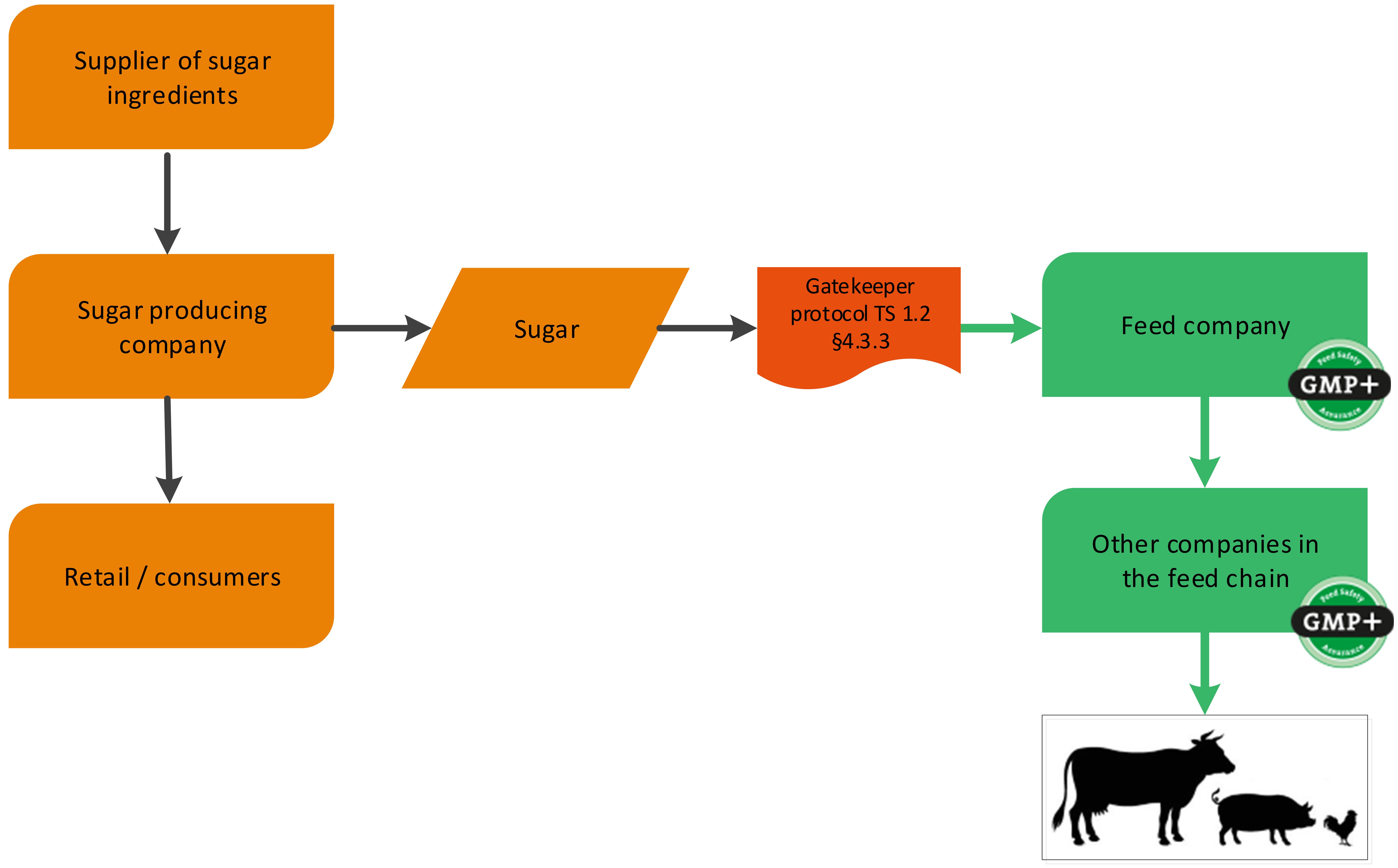
Flowchart 3: Foodstuff
4.1.2. The dairy industry
Dairy companies can produce several food grade products. During this production also by-products might be produced. A cheese factory is a foodstuff producing company (cheese) with a by-product which is used in the feed sector (whey). GMP+ certification begins at the cheese factory where the feed material whey is produced, see flowchart 4.
It could also be that a dairy company produces food grade milk powder that is purchased by a feed company. An example is shown in flowchart 5.
You can purchase dairy products in several ways:
- Dairy products, which are produced under EU Reg. 853/2004, can be purchased via TS1.2 Purchase 3.4.2;
- Dairy products, which are not produced under 853/2004, but under a GFSI-approved scheme, can be purchased via TS1.2 Purchase 4.3.3 (see flowchart 5 as an example);
- Dairy products, that are neither under GFSI nor under EU Reg. 853/2004 are classified as former foodstuffs and can be purchased via TS1.2 Purchase 4.3.4.
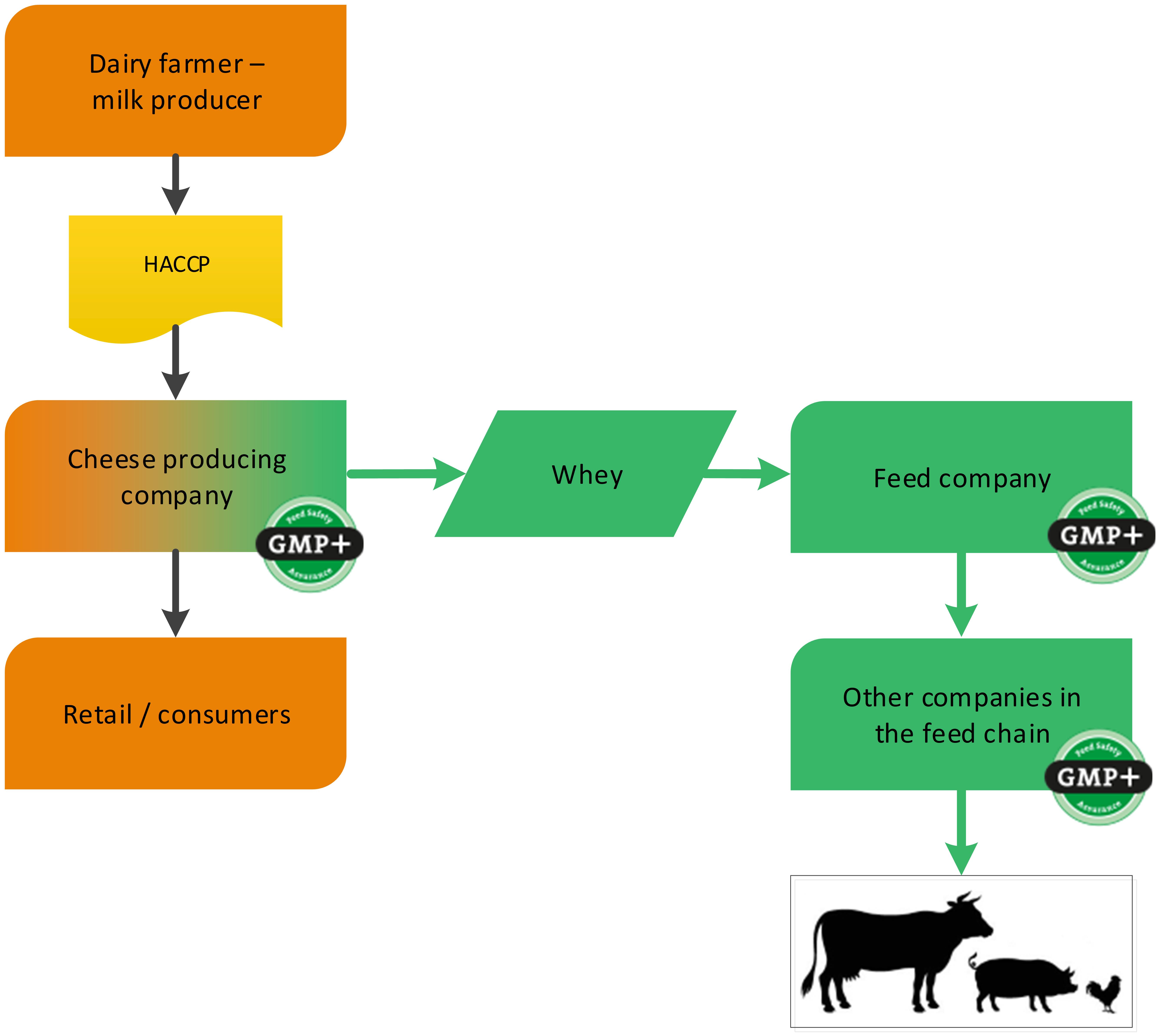
Flowchart 4: The dairy industry
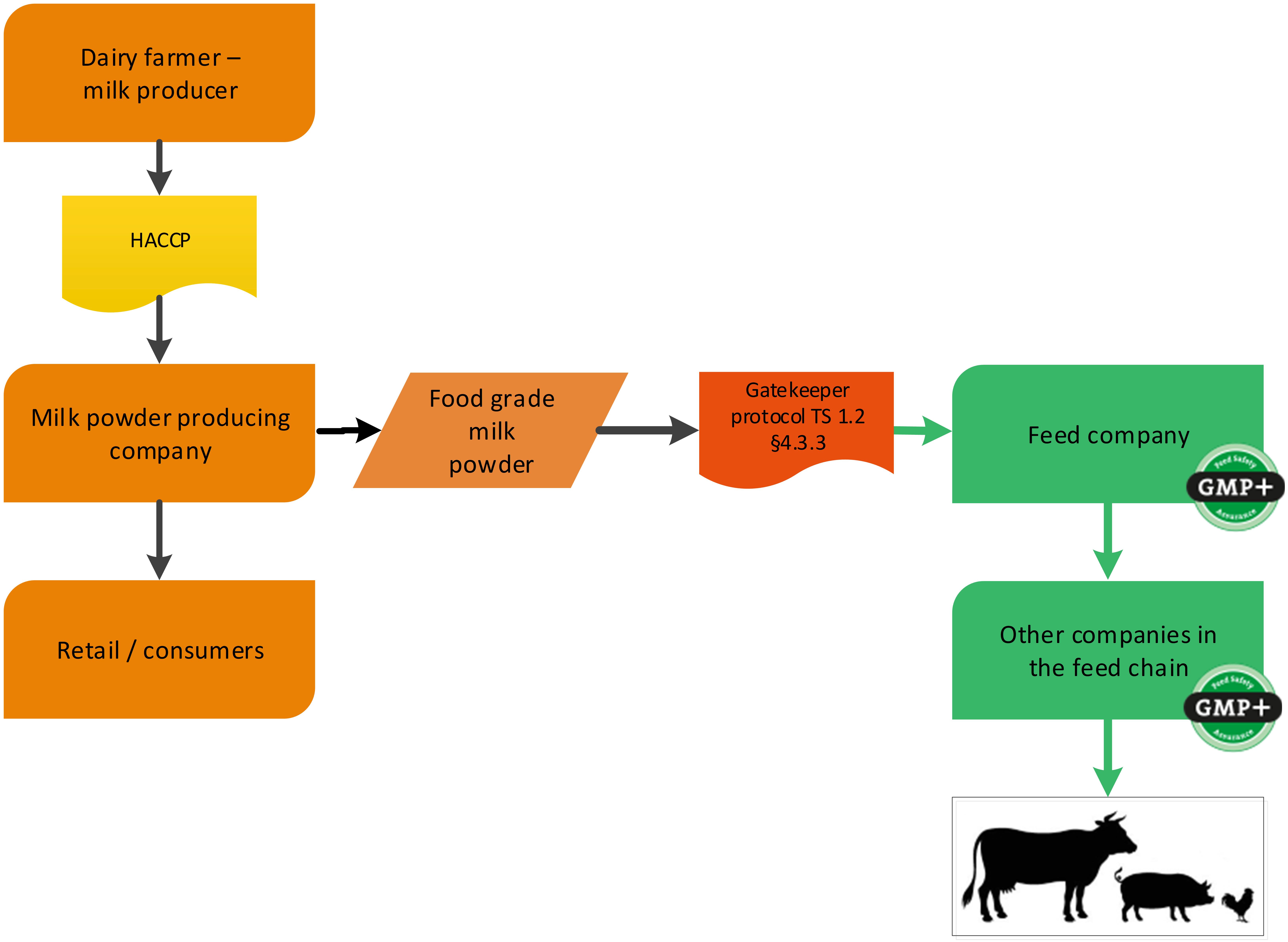
Flowchart 5: The dairy industry
4.1.3. The grain industry
4.1.3.1. By-products of the processing of grains, seeds and legumes
Grains, seeds and legumes are often used in the production of food. During the processing of grains, seeds and legumes, by-products are created which are used in the feed sector (for example wheat middling’s derived from a flour mill). GMP+ certification begins at the company where the feed material is produced, see 2 examples, one about the purchase of wheat and one about the purchase of rice, see flowcharts 6 and 7.
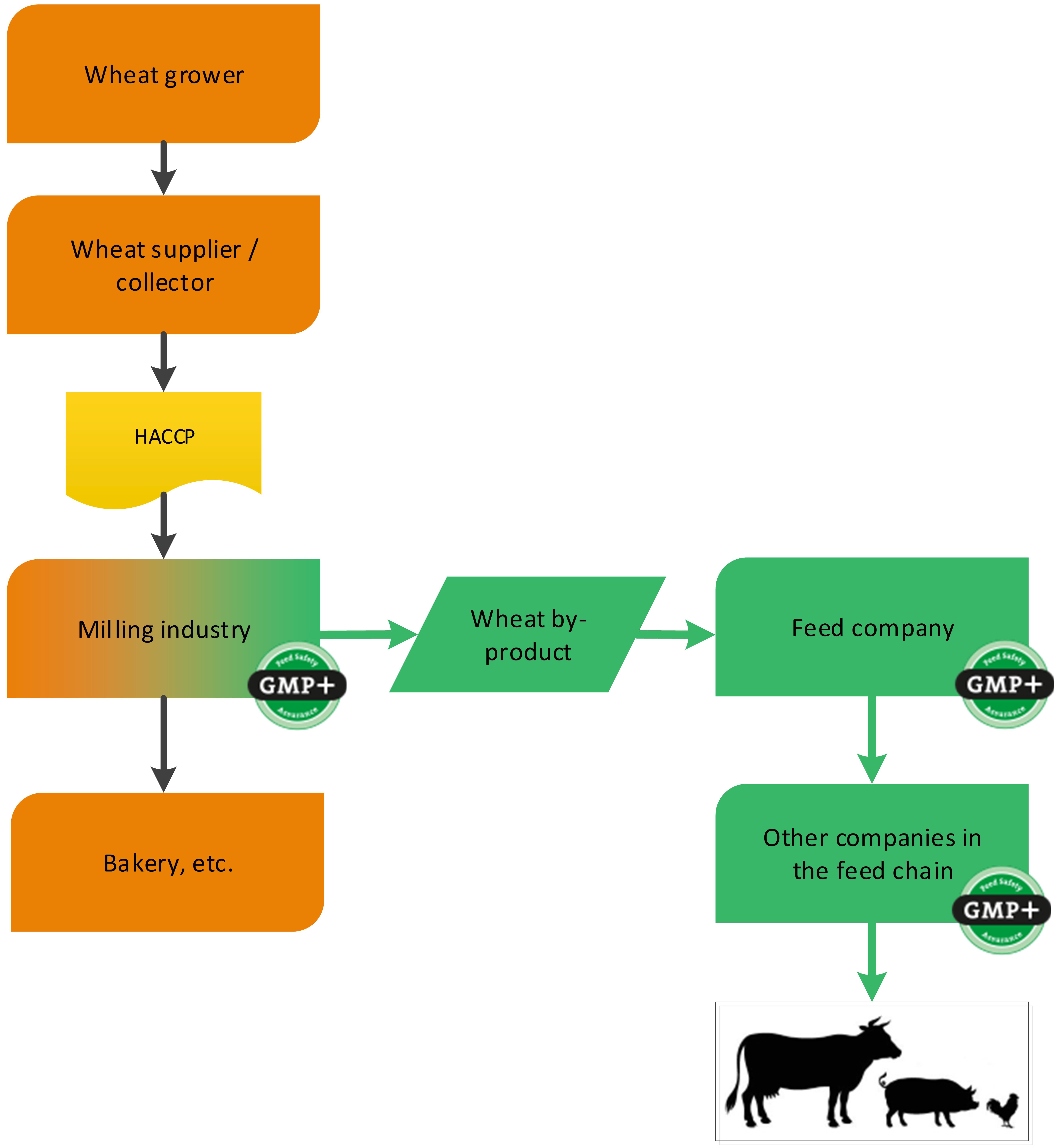
Flowchart 6: By-products of processing of wheat
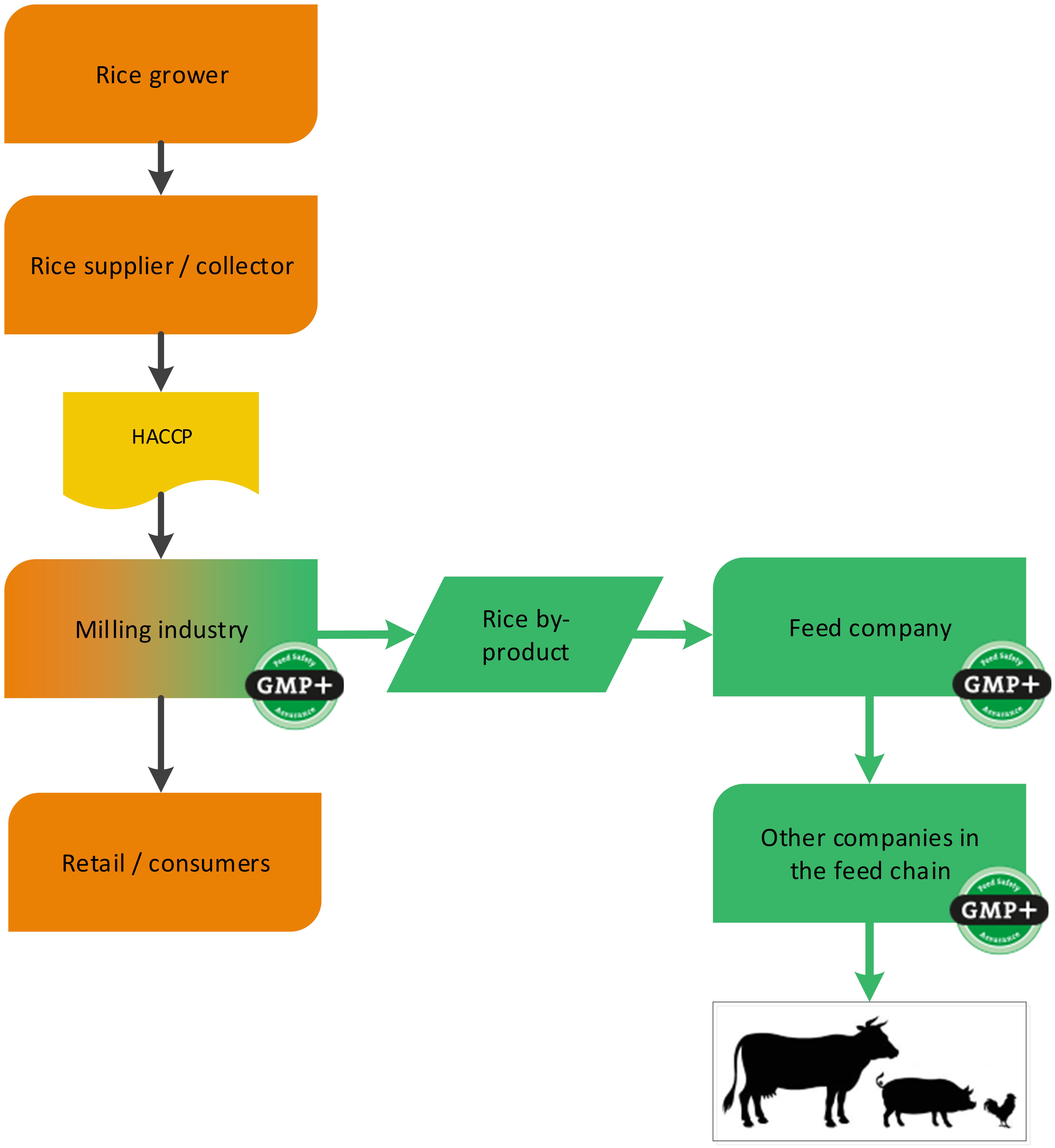
Flowchart 7: By-products of processing of rice
4.1.3.2. The bakery sector
Bakeries can also produce by-products and these can be delivered as feed materials. GMP+ certification is applicable from the step that the by-product is produced, see flowchart 8. This can be by-products originating from the production process, or can be returned products from supermarkets or bakery shops.
Note: The ingredients for the bakery do not have to be purchased from GMP+ certified suppliers.
The returned products may originate from their own bakery or from third parties. In the event of returns from third parties, it should be clear that these returned products were also originally produced under a HACCP system.
Also former foodstuff can be produced within the bakery industry, for example broken cookies. See flowchart 9 for an example.
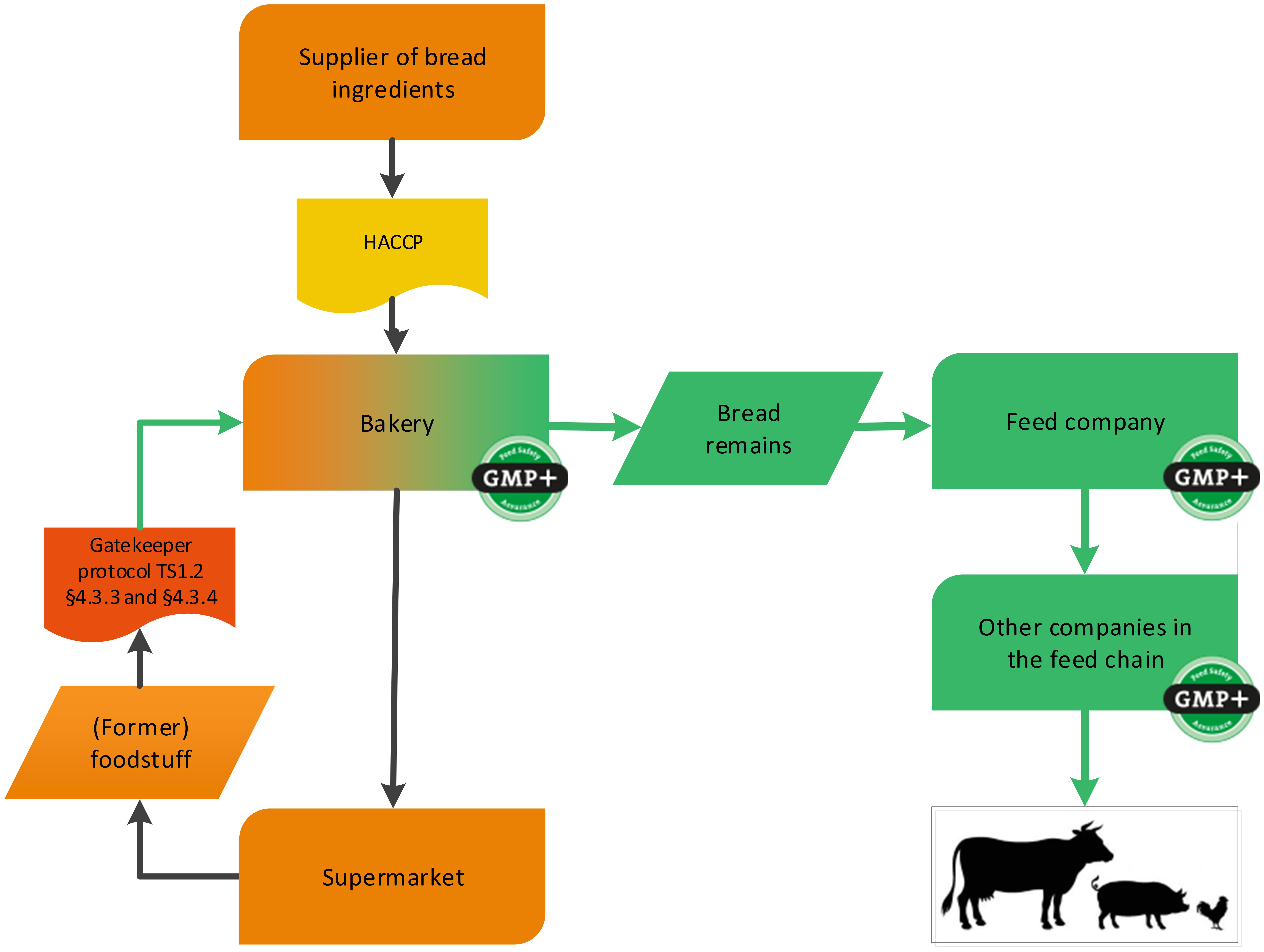
Flowchart 8: Bakery sector
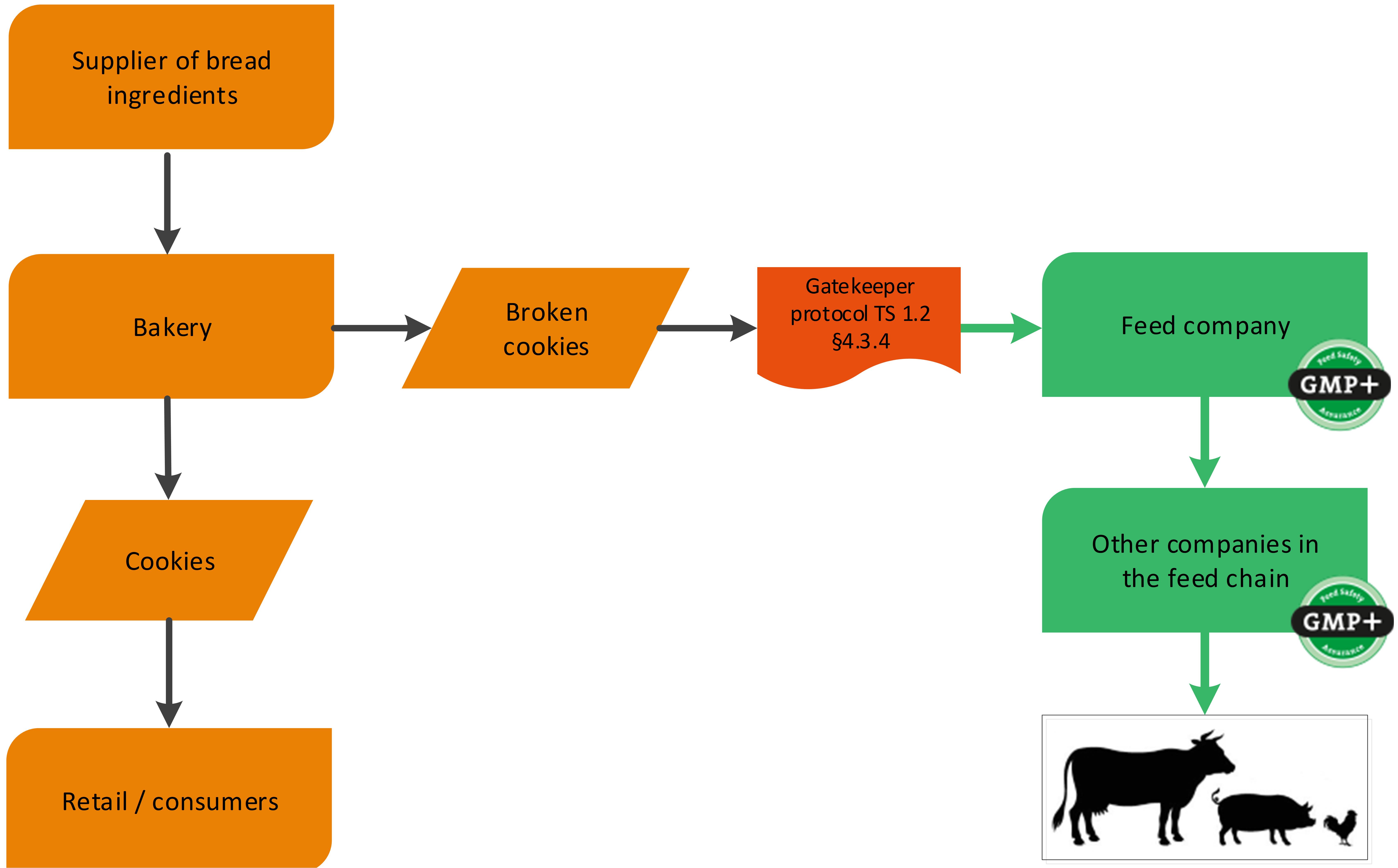
Flowchart 9: Former Foodstuff
4.1.3.3. The oil seed processing industry
Various by-products from the oil seed processing industry can be designated as feed (such as expellers or meal). GMP+ certification begins at the crusher at the moment when the feed material is produced, see flowchart 10. Also from refinery industry, feed materials can be created as a by-product.
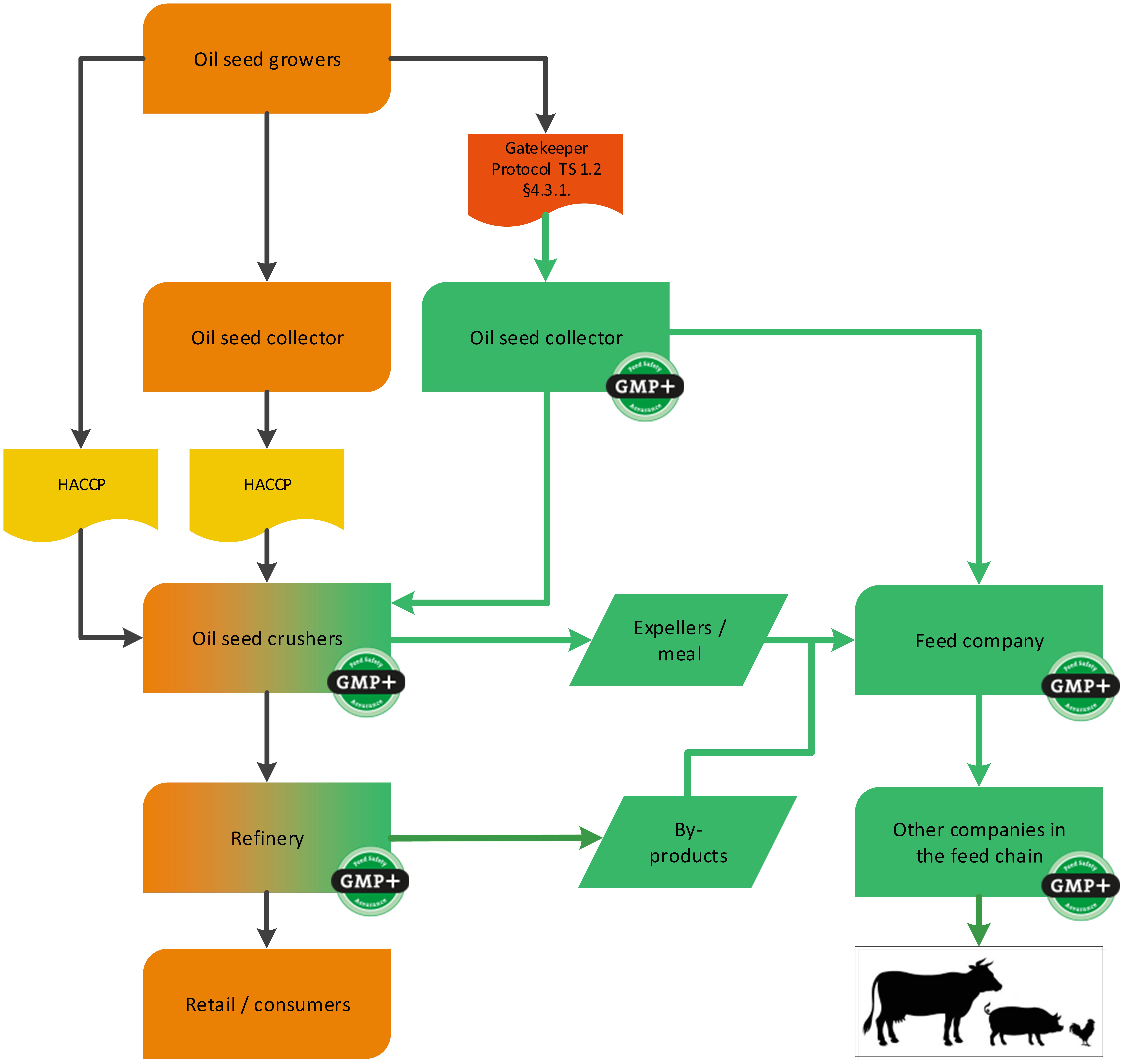
Flowchart 10: Oil seed industry
4.1.3.4. The potato grading industry
A potato grading company is grading/sorting potatoes for the food industry. Potatoes who will not be used in food (too much of not correct size for example) can be used in feed. They can be purchased as former foodstuff by a GMP+ certified company. For an example of this chain see flowchart 11.
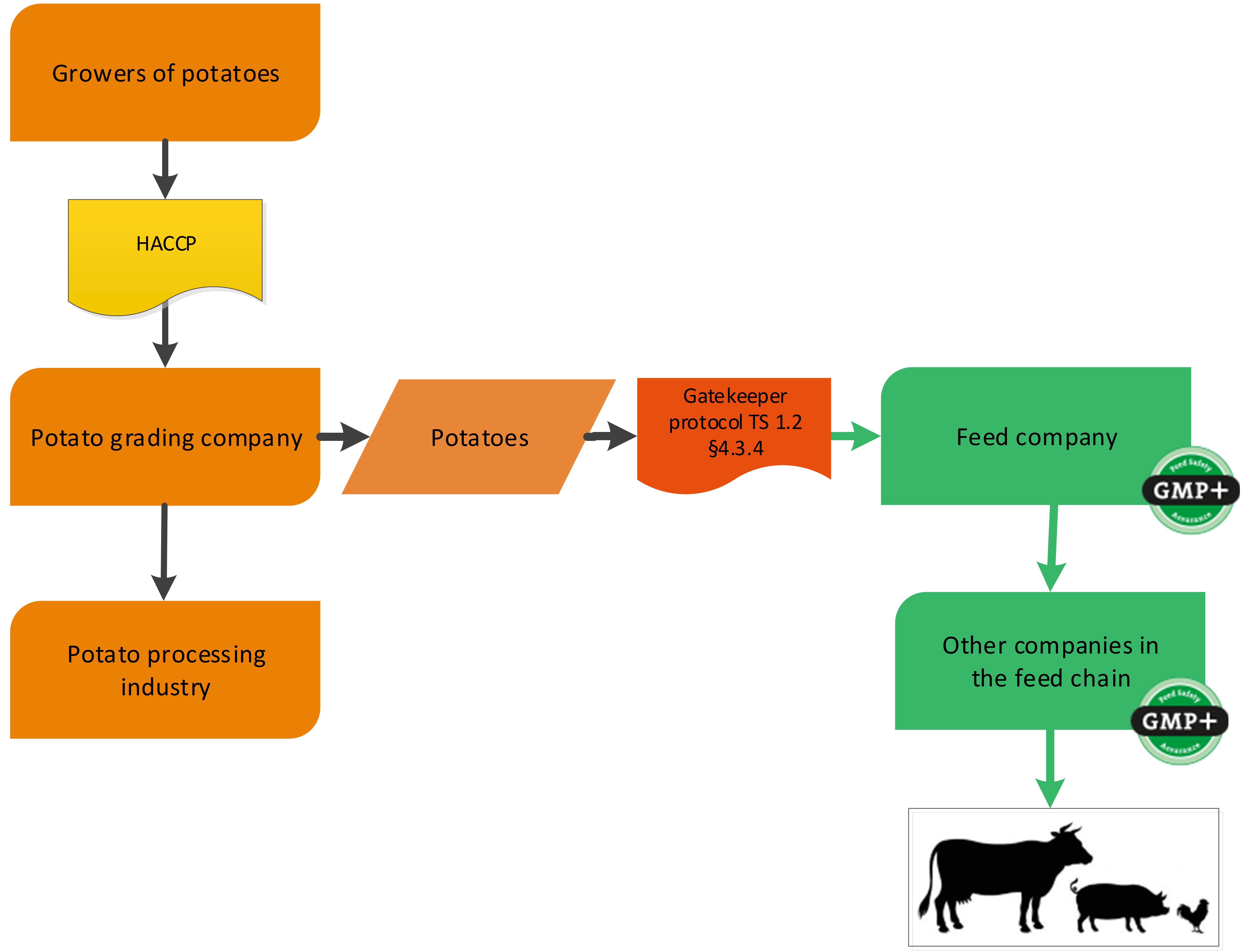
Flowchart 11: Potato grading industry
4.1.4. Fruit and vegetables
4.1.4.1. Fruit and vegetables from processing companies
Fruit and vegetables can be designated as feed at various different moments. For example at a processing company where a by-product (such as cut-outs) is created which is designated as feed. The GMP+ supply chain starts at the processing company where these by-products are created, see flowchart 12.
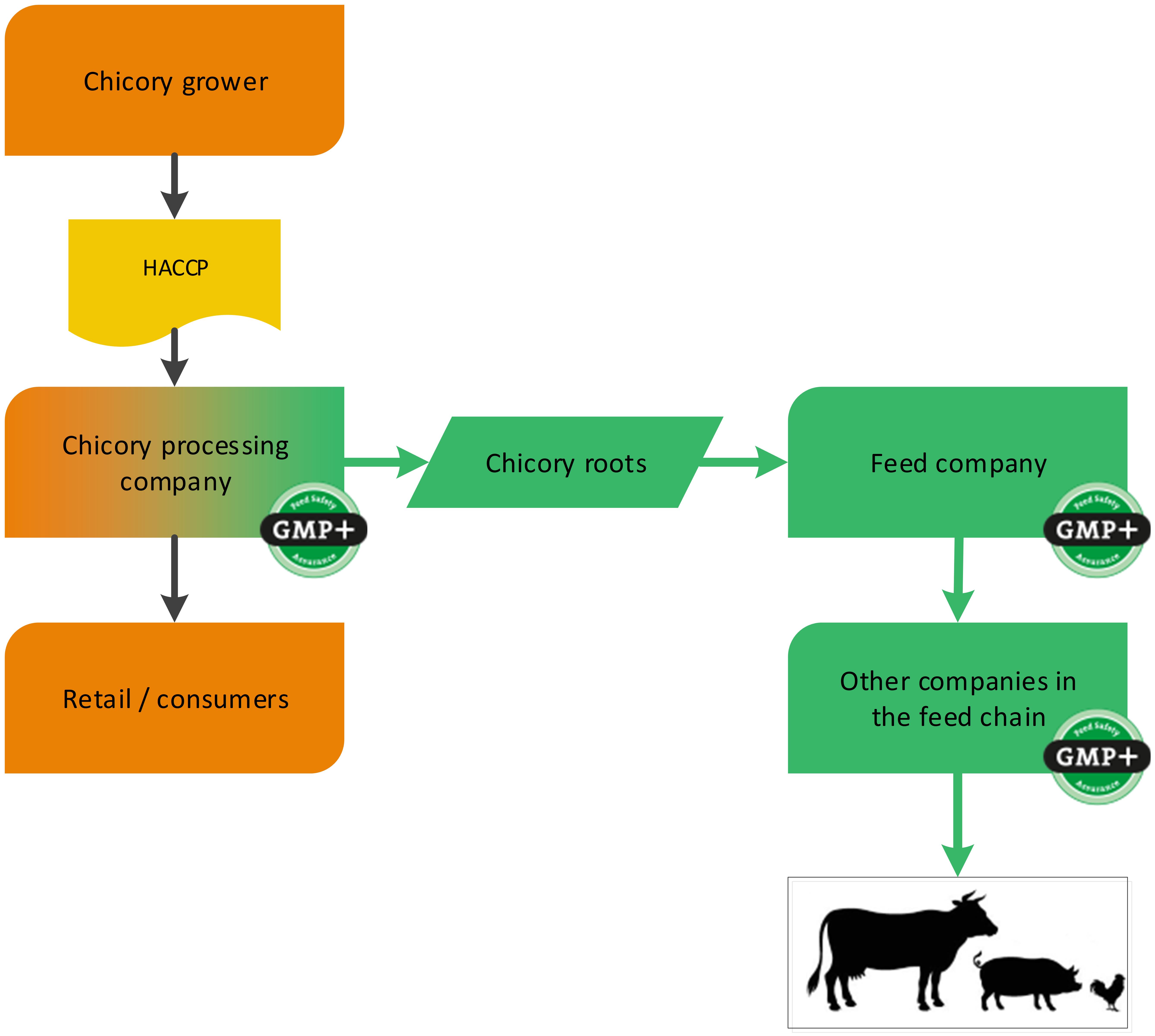
Flowchart 12: Chicory industry
4.1.4.2. Fruit and vegetables from the auction
Unprocessed fruit and vegetables from an auction can be purchased by a GMP+ company. The company performing the auction does not have to be GMP+ certified. The company which is purchasing the unprocessed fruit and vegetables is considered the first link of the GMP+ supply chain and therefore GMP+ certification is required, see flowchart 13.
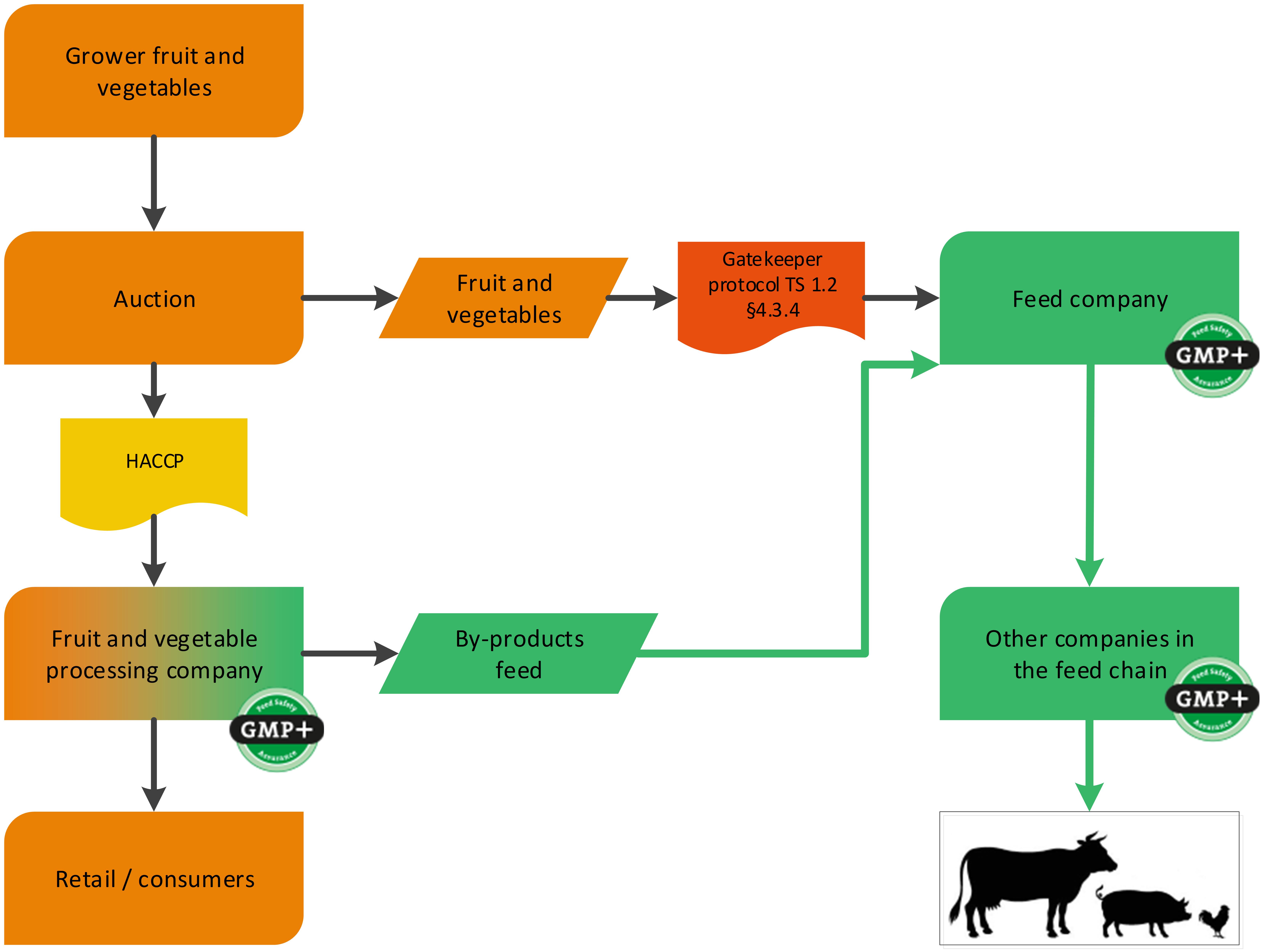
Flowchart 13: Fruit and vegetable from an auction
4.1.5. Processors of animal by-products
This industry can be split into animal by-products from the slaughterhouse and the fishing industry. See the next two paragraphs for examples of both.
4.1.5.1. Animal by-products from slaughterhouses
Animal by-products can be designated as feed based on legislation. For more information about legislation please see S9.5 Legislation and relation to GMP+ FC scheme. Only products which are classified as so-called Cat. 3 material can be designated as feed.
GMP+ certification begins at the point of the processor of Cat. 3 material, see flowchart 14. That is the point where animal by-products are produced that are used as feed.
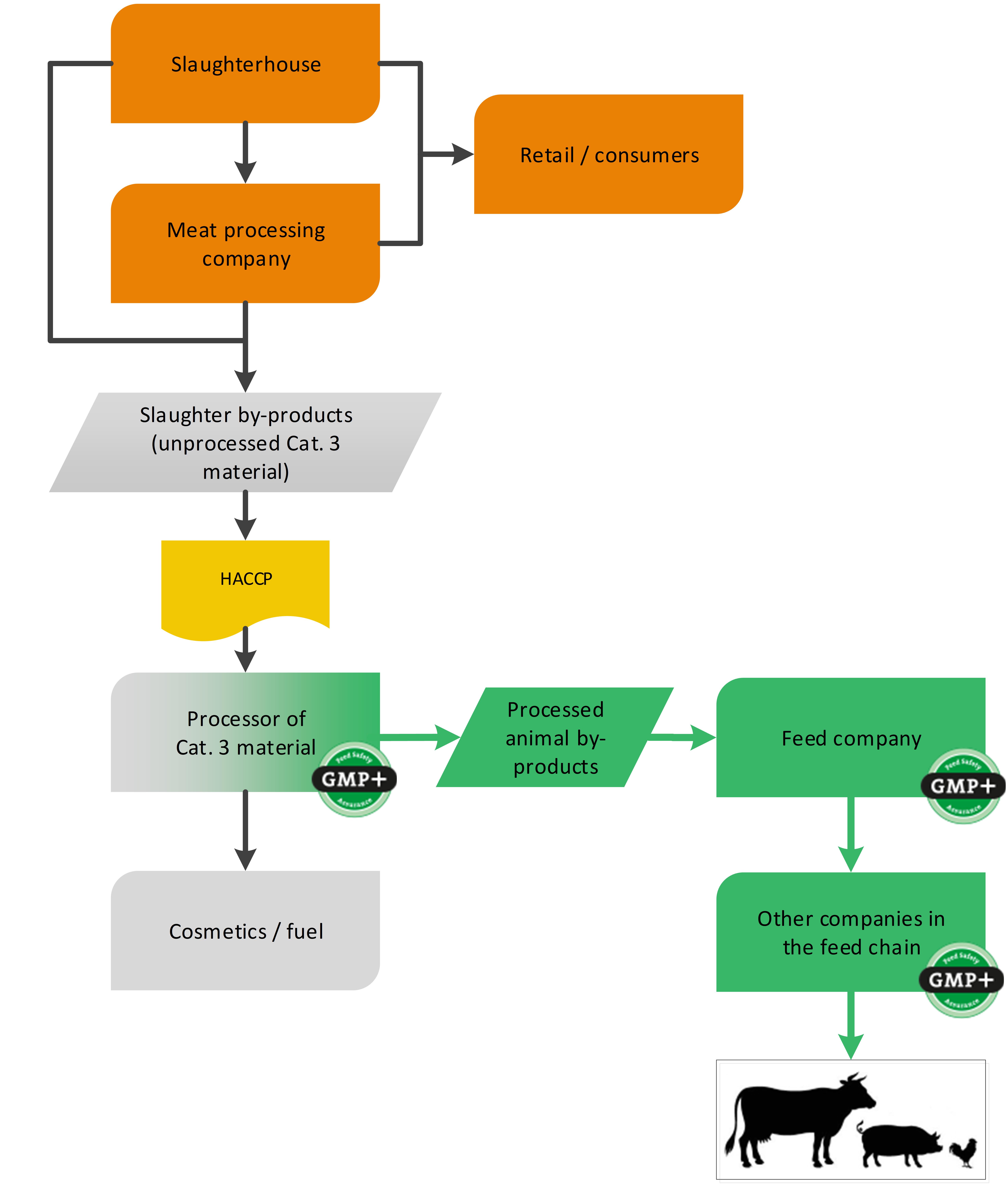
Flowchart 14: Processors of animal by-products
4.1.5.2. Animal by-products from fish
By-products from fish such as fish meal and fish oil are commonly used in the feed supply chain. The fish meal and fish oil producer is supposed to carryout a proper hazard analysis and do an entry check of its raw materials. See flowchart 15 for an example of the supply chain.
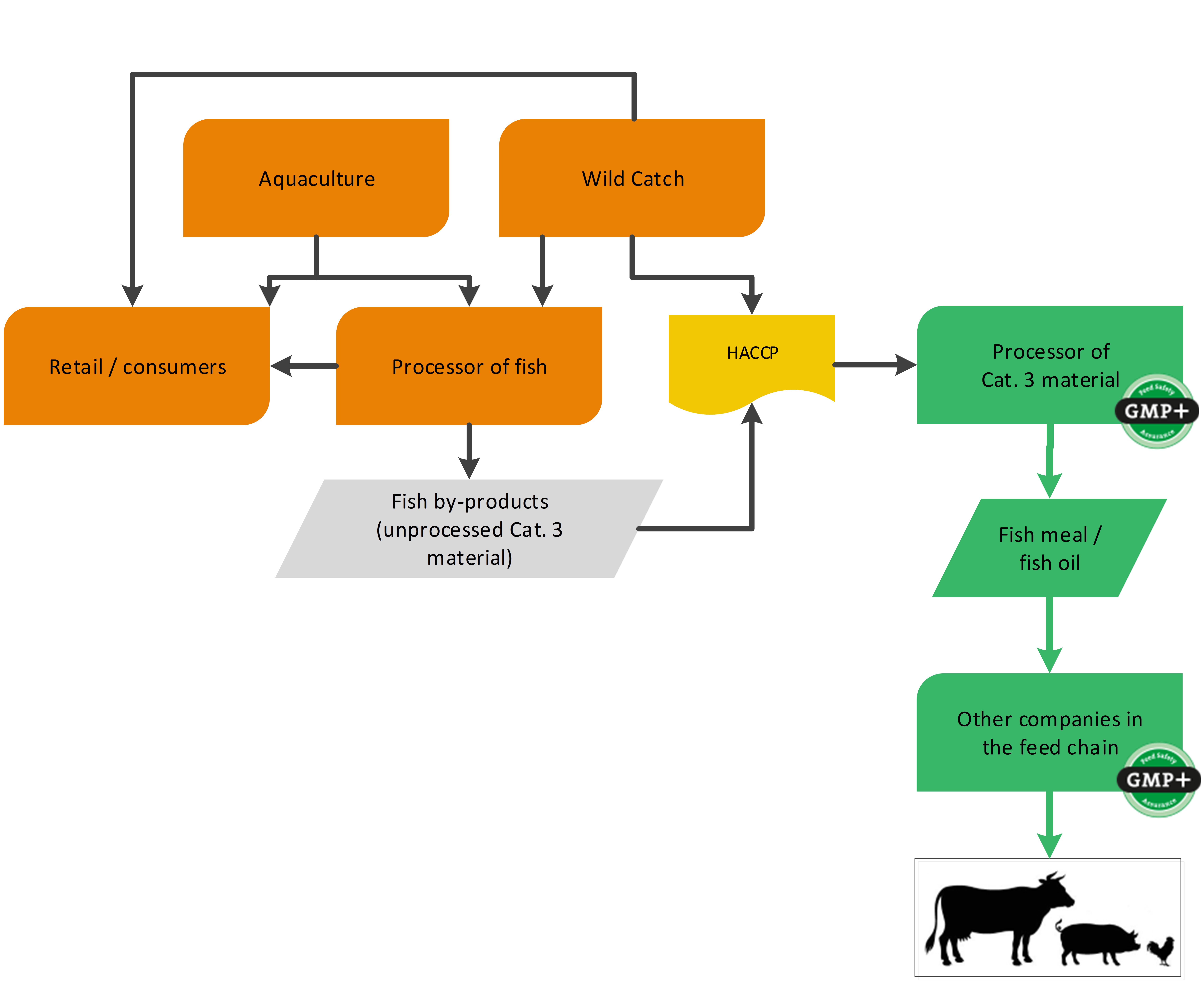
Flowchart 15: Processors of fish
4.2. Seaweed processing industry
A seaweed processor is a company that produces products which can be used in the feed sector (for example seaweed meal). GMP+ certification begins at the seaweed processor where the feed material is produced, see flowchart 16.
The seaweed does not have to be purchased from GMP+ certified harvesters (in case of seaweed harvested from the sea) or grower (in case of seaweed grown by a farmer).
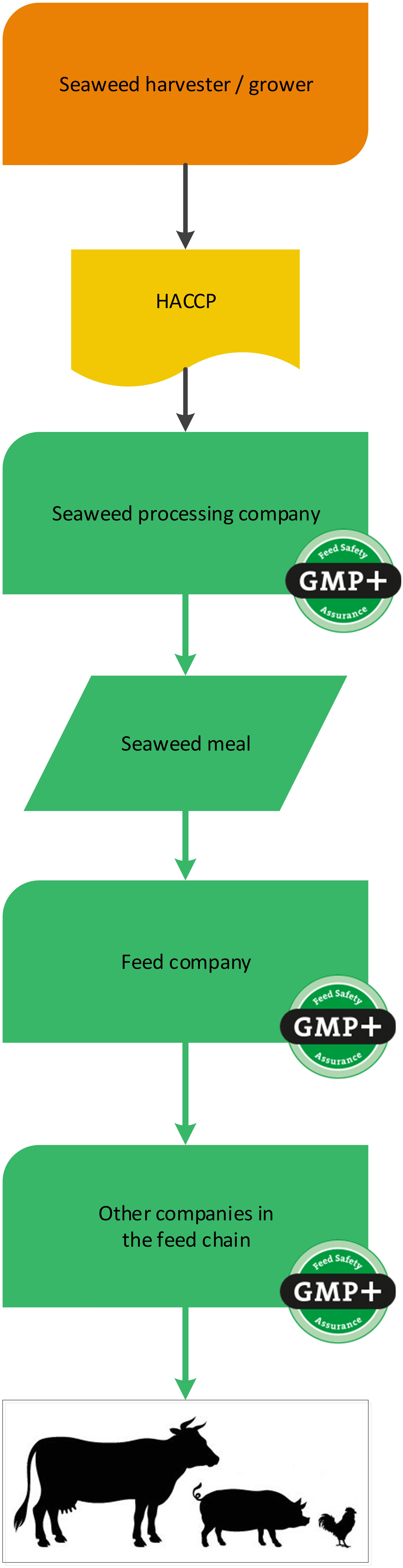
Flowchart 16: Seaweed processing industry
4.3. Unprocessed grains, seeds and legumes
Unprocessed grains, seeds and legumes can be purchased via a gatekeeper protocol. Within the GMP+ purchasing requirements, there are three options to use a gatekeeper protocol, see flowchart 17. Below the flowchart an explanation of all 3 options is given.
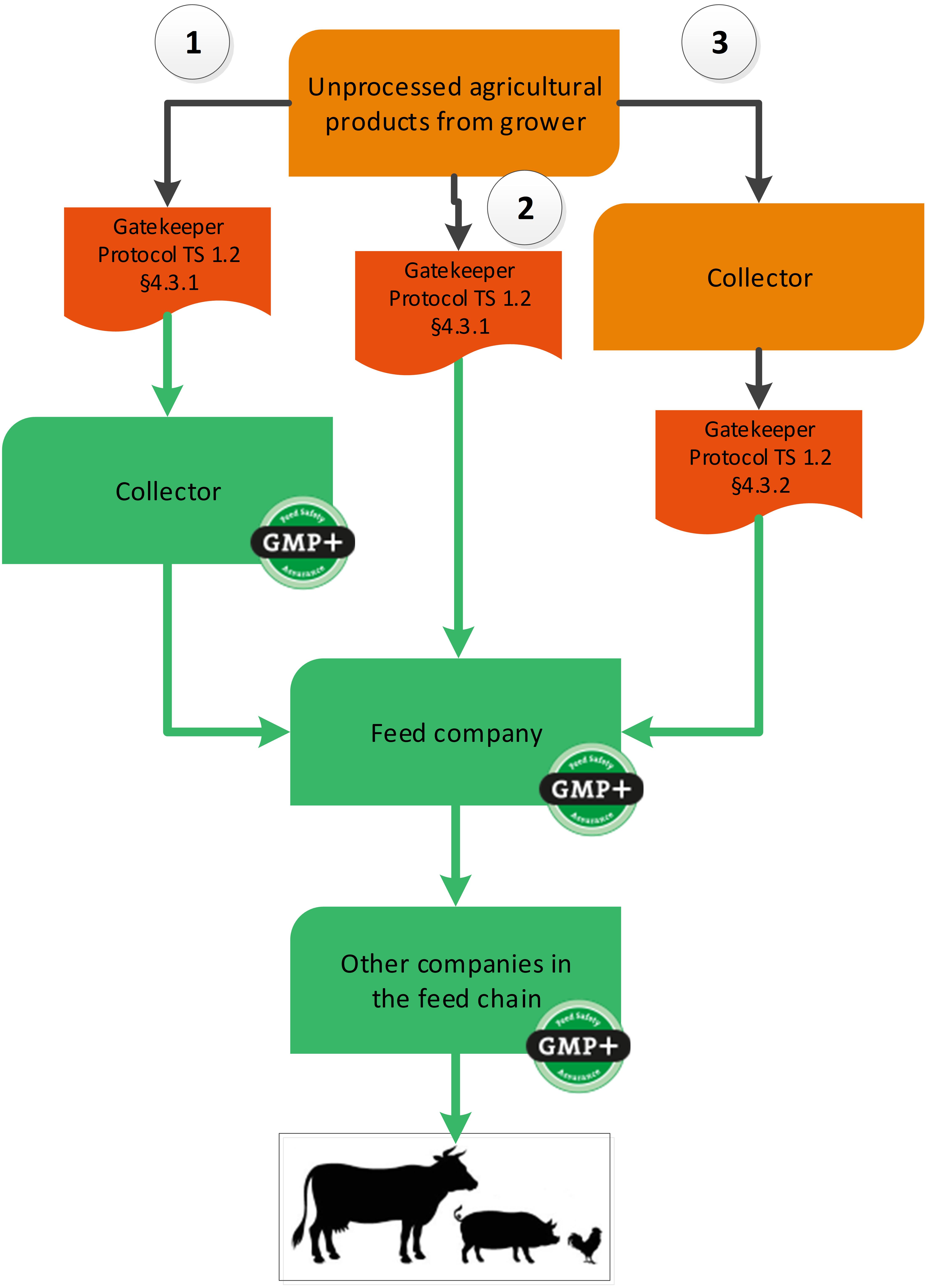
Flowchart 17: Unprocessed grains, seeds and legumes
Option 1: Collectors that purchase directly from the grower
As there are no GMP+ certified growers (these companies are not included in the scope of GMP+ certification), a collector purchases to buy unprocessed grains, seeds and legumes directly from a non-certified grower. In this case the collector that purchases these products directly from the grower needs to be GMP+ certified and needs to implement the gatekeeper requirements.
When a collector purchases grain with an unknown purpose (either food / feed / fuel), the collector should make sure that products comply with requirements of the applicable gatekeeper protocol TS1.2 Purchase 4.3.1. When the collector does not comply with requirements, it cannot sell products to GMP+ certified companies.
Option 2: Feed companies purchase from the grower
Feed companies can directly purchase unprocessed grains, seeds and legumes from growers without a collector. The feed company needs to follow the same gatekeeper protocol and requirements as described in option 1.
Option 3: Feed companies that purchase from an uncertified supply
It is also possible to purchase unprocessed grains, seeds and legumes a collector that has no GMP+ certificate. The company that purchases these products directly from the collector needs to be GMP+ certified and needs to implement the gatekeeper requirements.
When a company purchases grain with an unknown purpose (either food or feed), the company should make sure that products comply with requirements of the TS1.2 Purchase 4.3.2. When the company does not comply with requirements, it cannot sell products to GMP+ certified companies. This gatekeeper protocol is limited to certain countries of origin where the product is grown.
4.4. Biofuels sector
Also from the production process of biofuels, certain by-products are produced which are designated for feed. Because in this case this is not a foodstuff producing company but a technical factory, the application of the HACCP system is not legally mandatory for the biodiesel manufacturer or its previous links. GMP+ certification begins at the process step where by-products are produced that are used as feed. The raw materials for the biofuel producer do not have to be purchased from GMP+ certified suppliers.
4.4.1. By-products from biodiesel industry
An example of the biofuel industry is the production of biodiesel from rapeseed. During the production of biodiesel, the by-products rapeseed meal and glycerin are created which can be designated as feed, see flowchart 18.
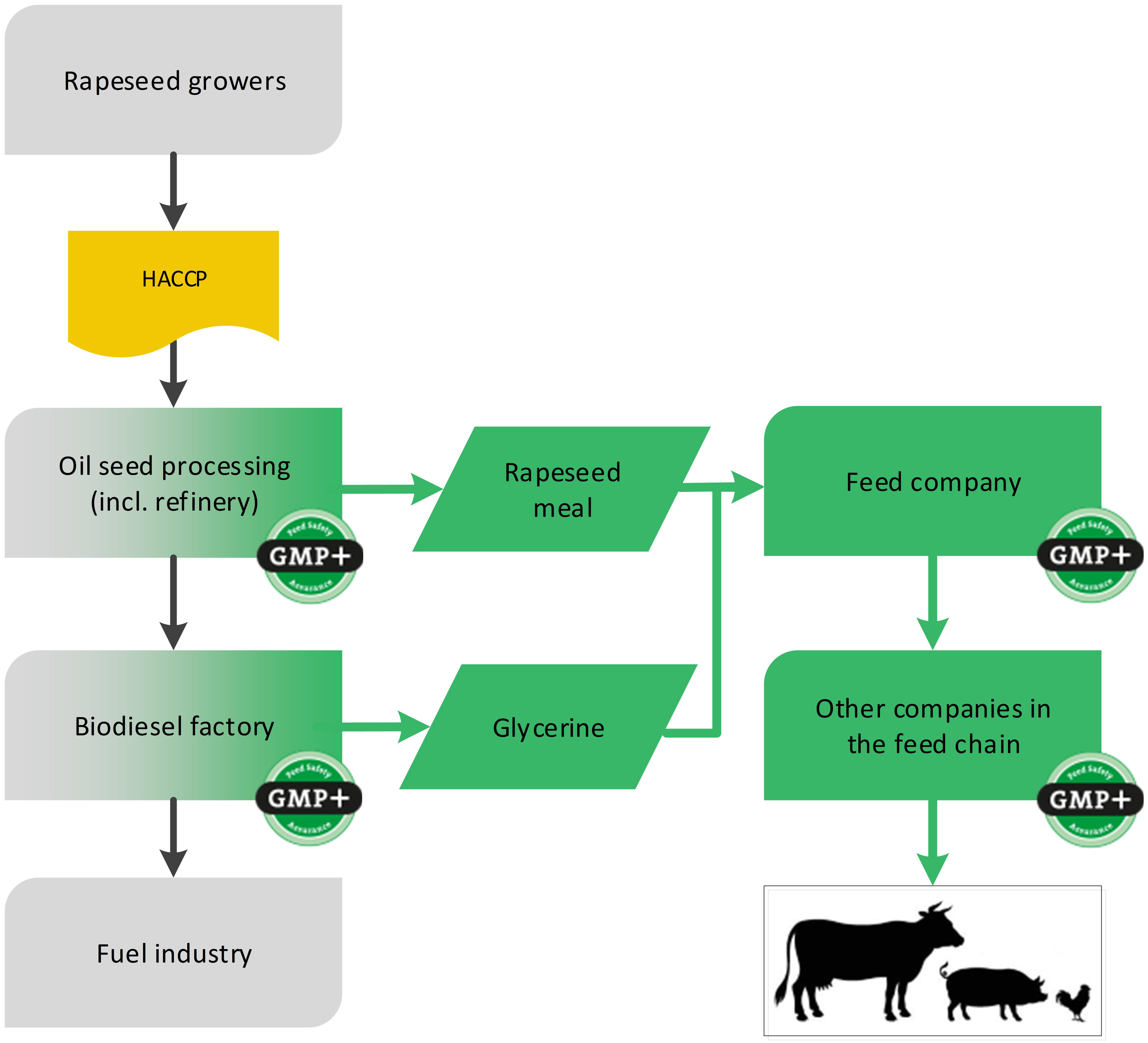
Flowchart 18: By-products of biodiesel industry
4.4.2. By-products from bio-ethanol
Another example of the biofuel industry is the production of bioethanol. During the production, the by-product distiller’s grain is created which can be designated as feed, see flowchart 19.
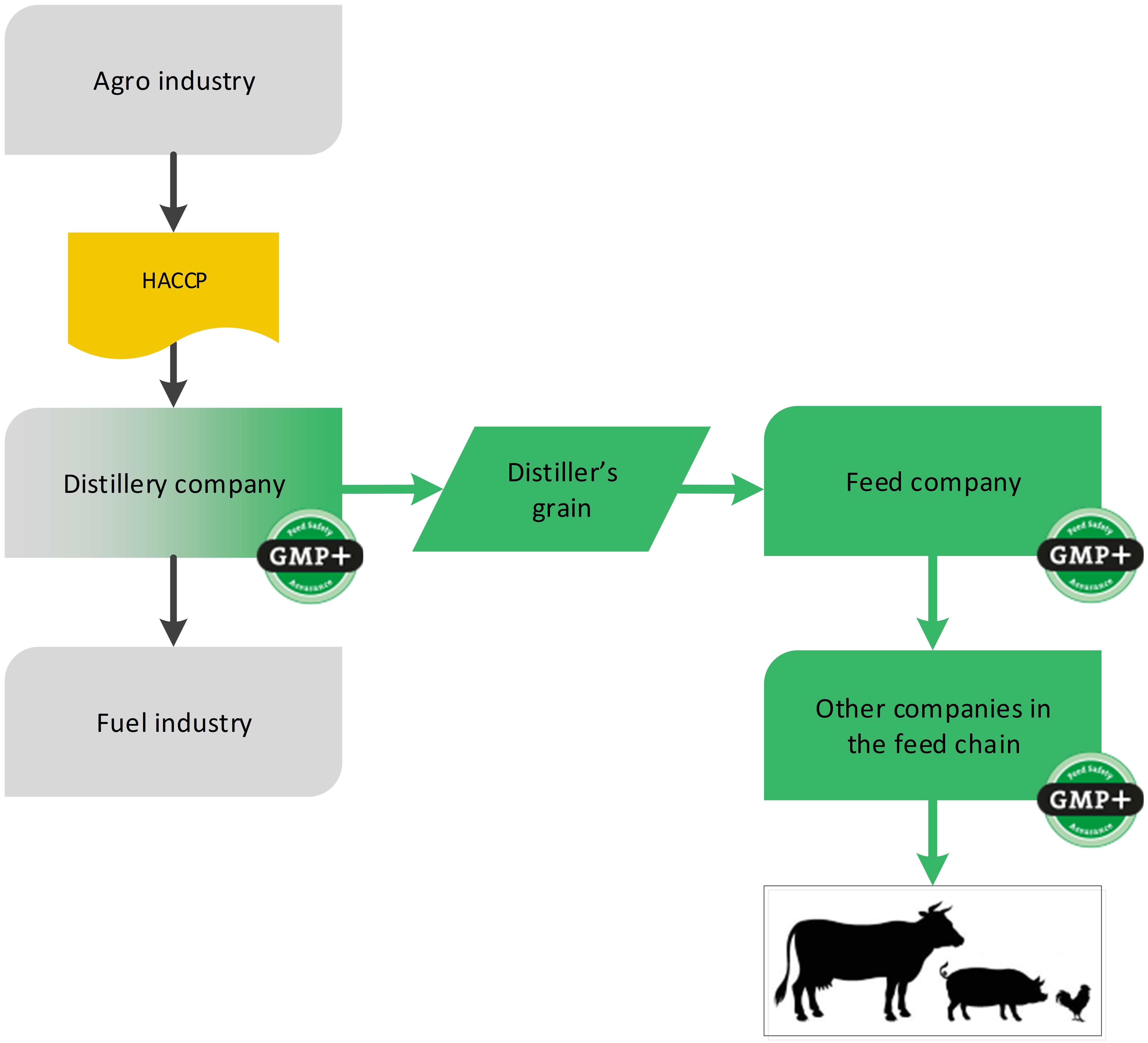
Flowchart 19: By-products of bioethanol industry
4.5. Feed additives sector
GMP+ certification can start at the company where a feed additive is produced for feed. But via a gatekeeper protocol it is also possible to buy feed additives from a non-certified supplier, see flowchart 20.
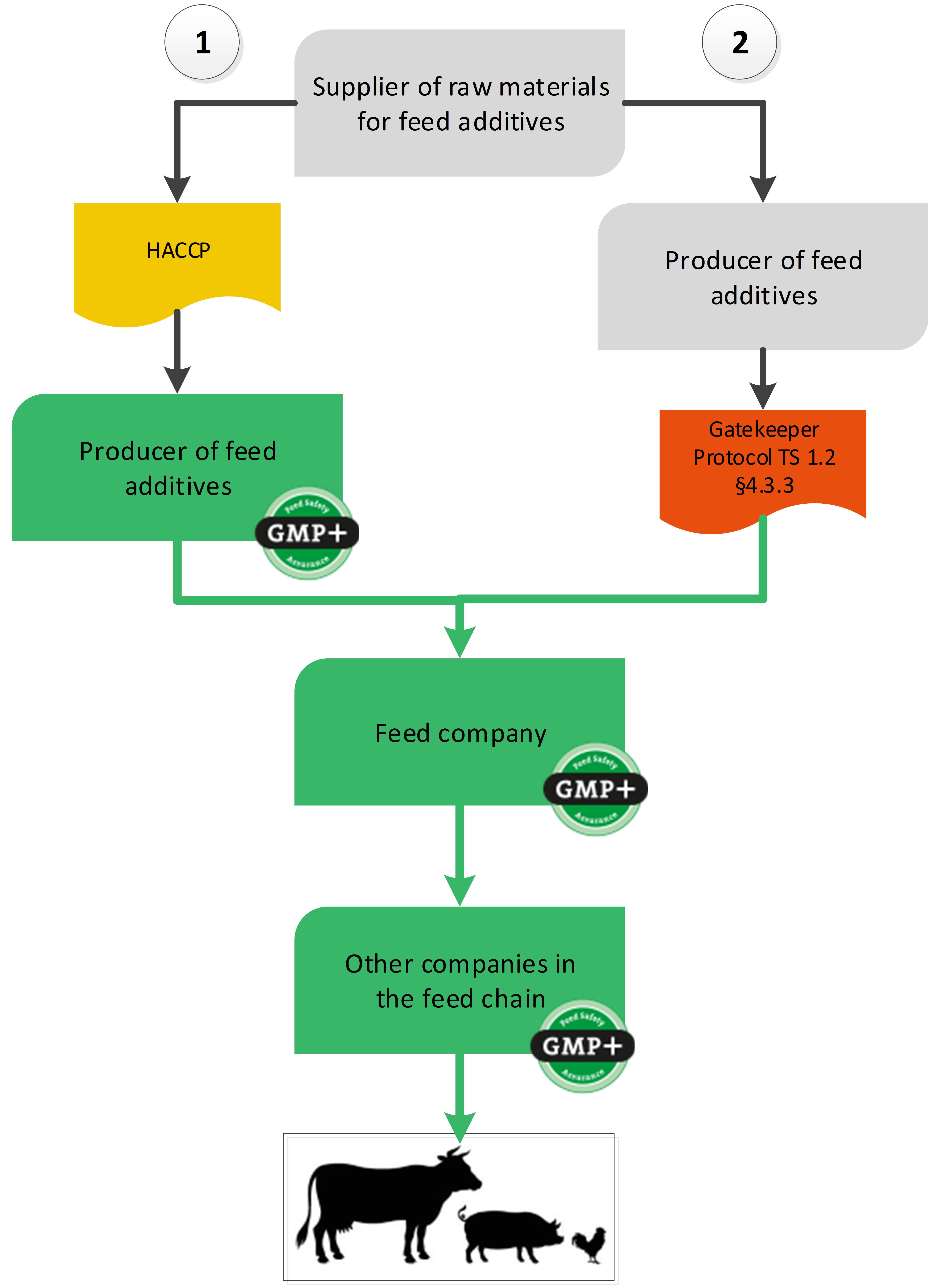
Flowchart 20: Feed additives sector
Option 1: Feed additive producer is GMP+ FSA certified
In this situation, the GMP+ certification starts at the point where the feed additive is produced. The scope of the GMP+ feed safety management system of the feed additive producer should include the production of the feed additives up to and including its sale to the feed company. The raw materials for feed additive production do not have to be purchased from GMP+ certified suppliers.
Option 2: Purchase from an uncertified supply chain
It is also possible to purchase feed additives from a completely non-certified supply chain.
The GMP+ certified feed company (for example a premixture company) is allowed to purchase feed additives from a non-GMP+ certified supplier. The GMP+ certified company should however comply with a number of Gatekeeper requirements. These are included in TS1.2 Purchase 4.3.3.
In both cases, the legal requirements for feed additives should be met. This means, for example, that for feed additives that are produced and / or sold on the European market, the European legislation applies. For more information about legislation please see S9.5 Legislation and relation to GMP+ FC scheme. All feed additives should be included in the European list of approved feed additives in order to be used within GMP+ certification.
4.6. Mineral feed materials (mines)
For the production of feed materials or feed additives sometimes raw materials come from mines. The products mined from the mines are not considered a feed material yet but a raw material which the GMP+ company processes into a feed material. Because the mine is not producing feed, GMP+ certification is not required.
Stones from a mine are only raw materials when they are not milled more than once and not dried. As soon as the stones are processed they will be considered as feed material and the company responsible for processing needs to become GMP+ certified. Because there currently are not a lot of GMP+ or equivalent certified supplier, there is a possibility for GMP+ certified companies processing mineral feed materials into their feed to request for an exemption for the purchase from non-certified suppliers.
The status of a product, given by the company, is not leading. GMP+ requirements, including GMP+ certification, are applicable when the product meets the description of a feed material based on the Feed Material Catalogue eg. (EG) 68/2013 and/or FSP Product list. European companies should also be registered according Reg. (EG) 183/2005.
It is possible that on one site the stones are mined and is processed into feed materials (production of feed materials) and a part will be sold for other purpose. The site then needs to become GMP+ certified for the feed material production process they carry out. The part of the mined products that is not processed can be sold as raw materials outside the GMP+ scope. Flowchart 21 is an example of the process and shows where GMP+ certification begins.
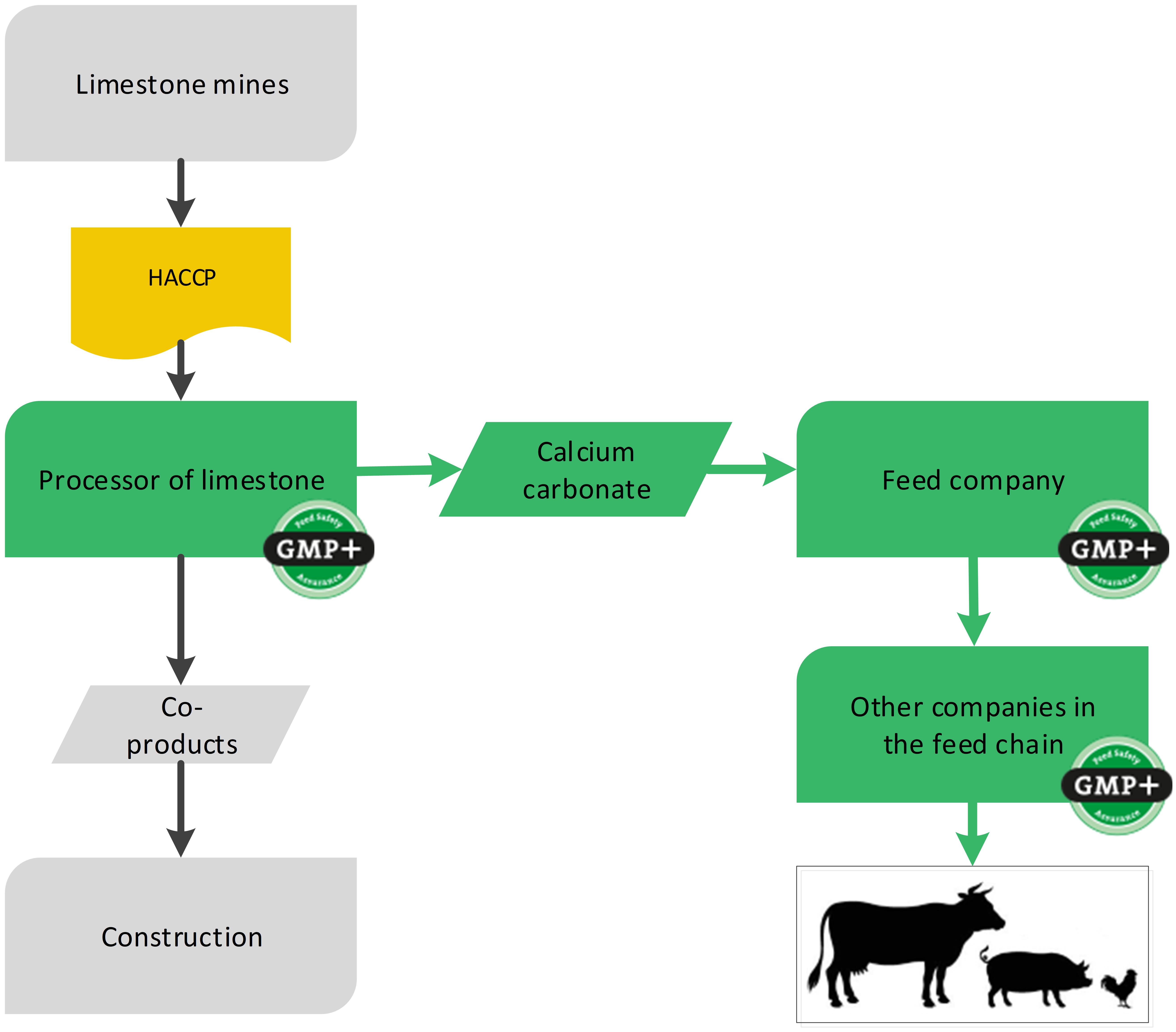
Flowchart 21: The mine industry
4.7. Producing feed materials out of other feed materials
In some cases a company produces a new feed material out of other feed materials (in this case to be considered as raw materials). The producer of the new feed material is to be considered as the starting point of the feed supply chain. The feed materials (‘raw materials’) which the company purchases, do not necessarily have to be purchased from certified companies. The original feed material needs to ‘disappear’ due to combined chemical reactions resulting in a new molecule structure.
This is not an option for companies who produce products from oils and fats as mentioned in GMP+ TS1.2 Purchase paragraph 4.2. In case of any doubts or questions please always contact GMP+ International to make sure the requirements are understood and used accordingly.
For the production of, for example, magnesium phosphate, the feed material magnesium oxide is used. The supplier of the magnesium oxide does not have to be certified for GMP+, but the producer of magnesium phosphate should carry out a proper hazard analysis according to the HACCP principles. Based on the results of this hazards analysis, and also on the quality assurance which is applied by the supplier, the GMP+ certified producing company will make a selection of suppliers and will adjust his (entry) check accordingly.
Note: The company that purchases the non-certified magnesium oxide cannot sell this as GMP+ certified feed material.
Flowchart 22 gives an example of the production of a feed material using a feed material as raw material.
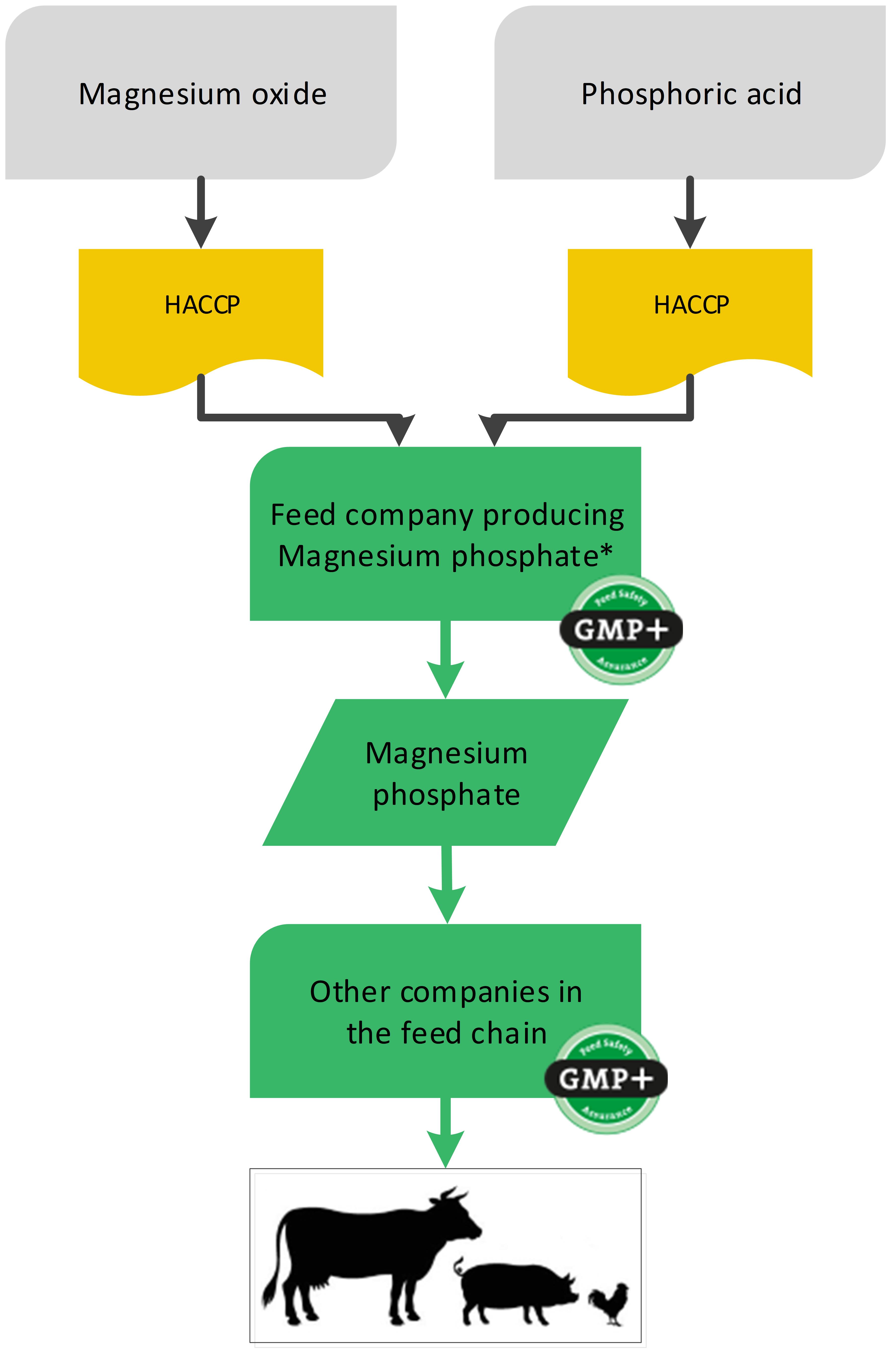
*The production includes chemical reactions, granulation and drying and therefore the molecule structure changes. The exact production process is written down in the Risk Assessment ‘Magnesium Phosphate’
Flowchart 22: Feed ingredients used as raw materials
4.8. Insects and insect products
In Europe it is allowed to use insects and insect products in feed. The companies who produce these insects and insect products are producers of feed materials. They need to assure the safety of the products and demonstrate as such with a GMP+ certificate.
The nutrition they purchase to feed their insects does not need to be GMP+ certified, HACCP principles are applicable. Neither do the mother insects used to breed the insects. The mother insects are not intended to be used as feed and only used for breeding, see flowchart 23.
For more information about this industry, see S9.41 Insects in feed.
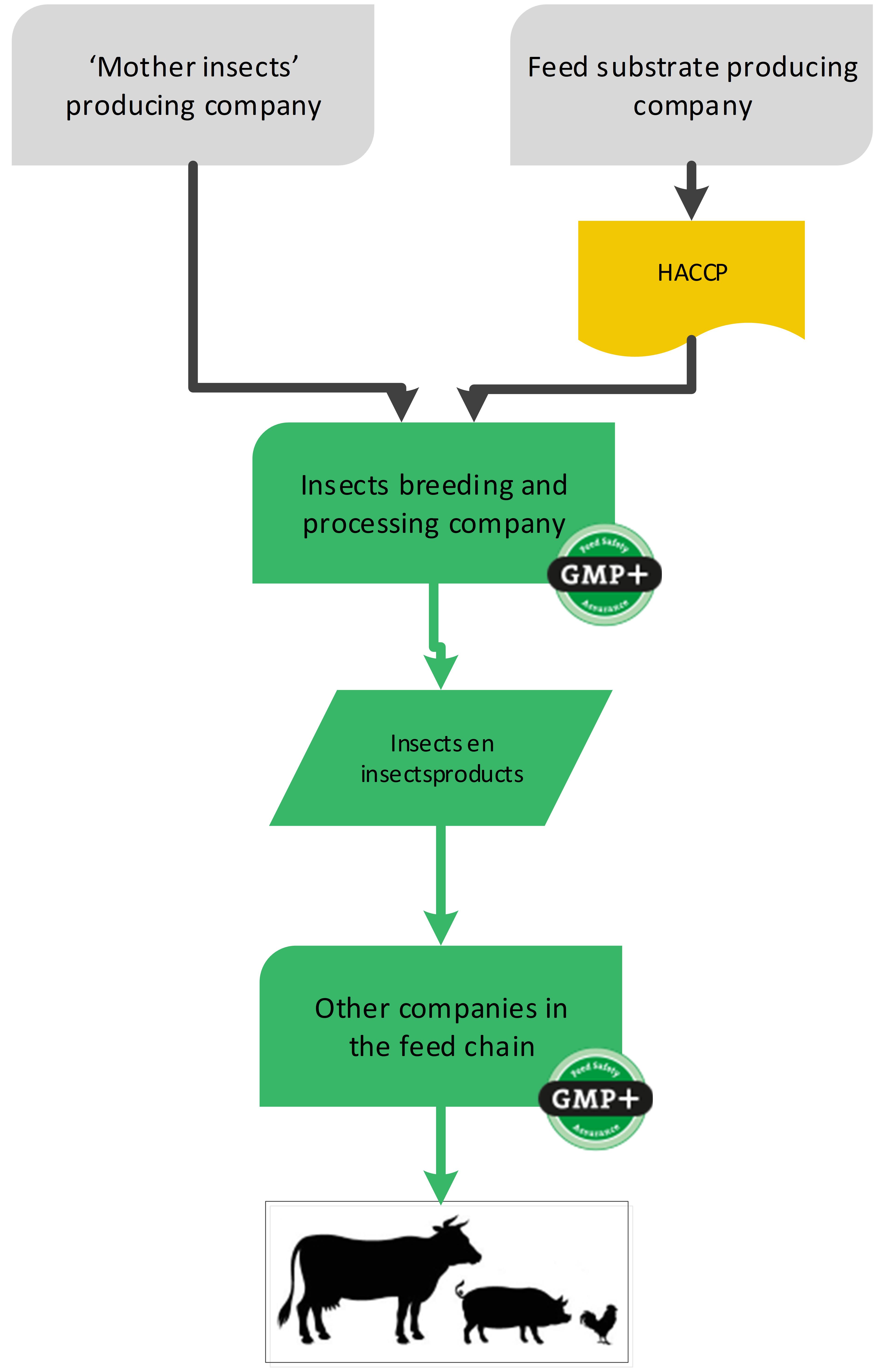
Flowchart 23: Insect producing industry
4.9. PO Box, Invoicing address, Registration address and Postal address
This flowchart explains the definitions of PO Box, Invoicing address, Registration address and Postal address in relation to certification. All definitions are listed in F0.2 Definitions.
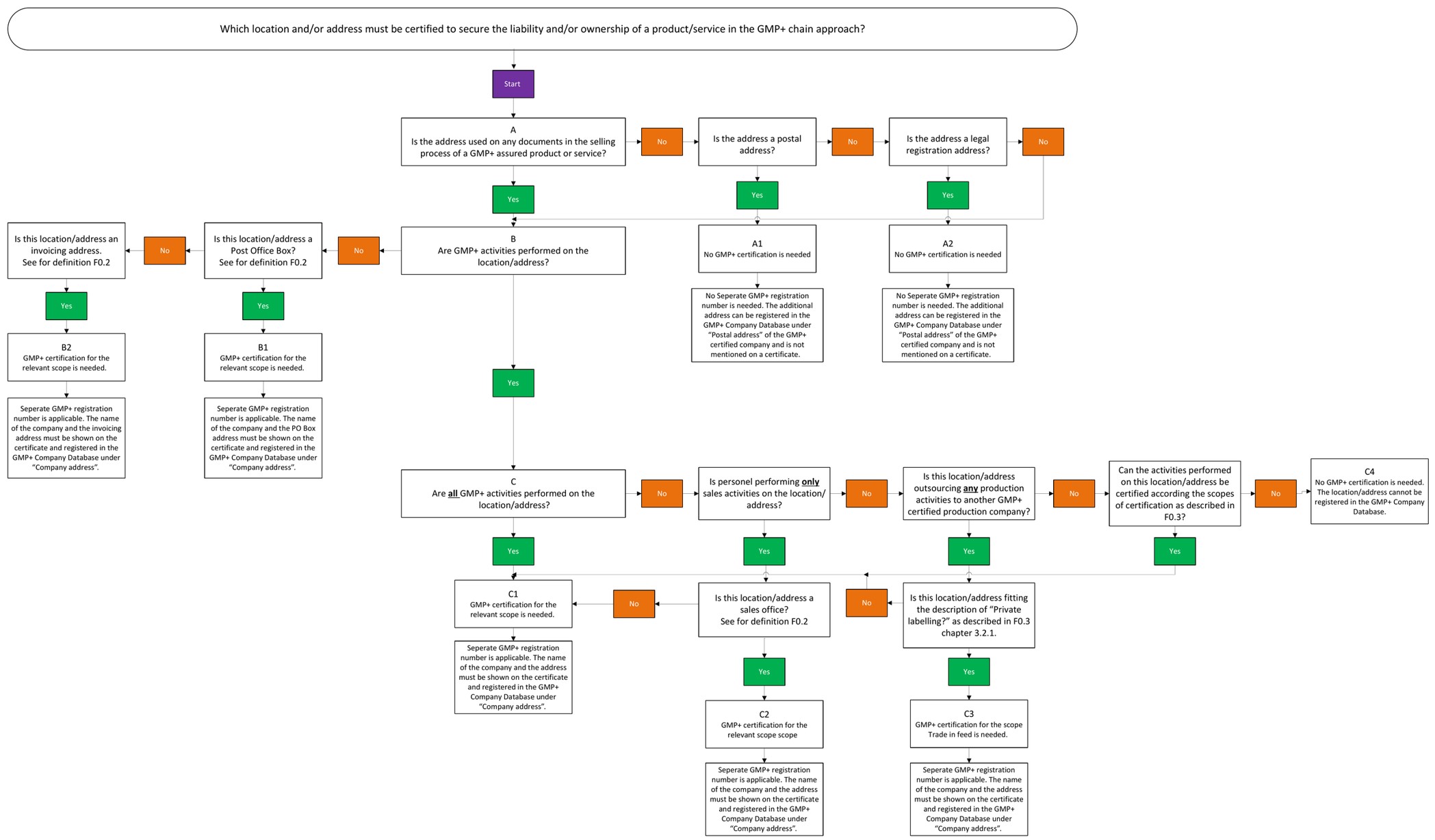
5. Frequently asked questions about purchase of feed and/or service
This chapter gives an overview of frequently asked questions about purchase based on the requirements in TS1.2 Purchase. The questions are categorized by general questions about purchase and questions about gatekeeping.
General purchase questions
What do you mean with ‘a certified company’ and with ‘a non-certified company’?
With a ‘certified company’ we mean a company which is certified according to the GMP+ FC scheme or another, accepted feed safety scheme For a complete list of accepted feed safety scheme go to the TS1.2 document.
With a ‘non-certified company’ we mean a company which is not certified at all or is certified by a non-accepted feed safety scheme. For example a food safety certification scheme (e.g. BRC).
How should I read table 3.9 in TS1.2 Purchase with requirements for purchasing services?
Table 3.9 shows the purchase of a certain process step, eg bagging of compound feeds or drying of a feed material (subcontracting). It can also be a series of process steps, for example the complete production of compound feed. If you want to let another company do this, for example because they can do this very well, then that company must be certified. So you purchase these process steps from that company.
Does the storage of packaged goods at third parties need to be GMP+ certified?
Storage of packaged goods does not need to be purchased from a GMP+ certified service provider. This is stated in the note in chapter 3.5 of TS1.2. Protocol 4.4.3 must be applied.
Note: The option that you can purchase storage of packaged goods from a non-certified company must not be interpret as if you can exclude your own storage of packaged goods from the scope of your Feed Safety Management System and certification: own storage of packaged goods must be controlled with the Feed Safety Management System and be certified. Even if your packaged goods are stored on one of your own separate locations.
Does the transport of packaged* goods by an external company needs to be GMP+ certified?
Transport of packaged goods does not need to be purchased from a GMP+ certified transport provider. This is stated in the note in chapter 3.6 of TS1.2.
This means that a GMP+ company can make an agreement about transport of packaged goods with a transport company. The GMP+ certified company should take its own responsibility and apply HACCP-principles when selecting a transporter. He should lay down in an agreement all the rights and obligations of both parties about safe transport.
Note: The option that you can purchase transport of packaged goods from a non-certified company must not be interpreted as if you can exclude your own transport of packaged goods from the scope of your Feed Safety Management System and certification: own transport of packaged goods must be controlled with the Feed Safety Management System and be certified.
*See F0.2 for the definition of sealed containers.
Does production/processing done by a third party/subcontractor based on a contract need to be carried out by a GMP+ certified company?
a. When use is made of a third party/subcontractor to produce feed (so called toll manufacturer), both parties must be certified:
- the company giving order to produce feed must be certified with the scope Trade in Feed;
- the third party/subcontractor must be certified with the relevant GMP+ production scope or corresponding scope from other, accepted certification schemes.
This also applies to the third party/subcontractor which is certified for an approved food safety certificate (BRC, IFS, FSSC, SQF etc.). See TS1.2 Purchase 3.9
b. When use is made of a third party/ subcontractor only for a specific processing step (e.g. drying, spray-drying, packaging etc. by a specialized company) then the third party/ subcontractor does not have to be certified. However, the use of a third party/ subcontractor is only possible with approval from GMP+ International. The certified company must apply for an exemption.
I use a Country Note. Am I obliged to use the purchasing requirements from TS 1.2?
No, you are not obliged to do this.
As a company you have to make a choice.
- You either comply to the requirements of the Country Note;
Or
- You comply to the requirements of TS1.2.
How should I purchase food additives?
Food additives can be purchased under gatekeeper protocol 4.3.3 of TS 1.2 as foodstuff insofar as they have been produced under a GFSI recognized scheme.
Please note: when a food additive is used in feed it is legally to be considered as a feed additive or a feed material. This might have consequences for labelling, dosing and application in feed.
Gatekeeping questions
Can I use the gatekeeper protocol(s) for buying from a certified company?
No, you cannot act as a gatekeeper for purchasing feed products (e.g. feed materials) or feed services (e.g. transport or storage) from a certified company. This means, for example, that you cannot use the gatekeeper protocol to purchase feed products or service which another GMP+ certified company has excluded from its GMP+ certification. That GMP+ certified company must bring these products or services under the scope of his own certification.
There are 2 exceptions to this condition
- Protocol 4.3.2 ‘Purchase of unprocessed grains, (oil)seeds and legumes: This protocol allows you to purchase non-assured grains, (oil)seeds and legumes from another GMP+ certified company, when this GMP+ certified company sells these products to you on FOB-conditions.
- Protocol 4.3.8 ‘Purchase of processed feed materials’: In some situations, there is a trader in between you as gatekeeper and the non-certified producer. The GMP+ status of this trader does not matter if this trader sells on FOB-conditions to you.
Will companies covered under a gatekeeper protocol be registered within the GMP+ Company database?
No, companies, covered via any protocol, are not registered in the GMP+ Company database. As a receiver of a product or service, you must therefore take into account that you must ask the gatekeeper if necessary whether the relevant product or service is assured by him.
What requirements are applicable to allow purchase on FOB conditions concerning protocol TS 1.2 Purchase 4.3.8 Purchase of processed feed material?
- First, you have to find out if it is allowed to purchase the feed material as a gatekeeper. The gatekeeping protocol is not allowed to use for the purchase of specific feed materials-countries-combinations. See for this the table in the protocol. You can only buy the feed materials from certified companies.
- If you are allowed to use the gatekeeper protocol for purchase a feed material (so an ‘allowed product’), it might be that there is a trader in between (as an intermediate). The country where the trader is located does not matter if the trader is selling FOB to you. So, even if this trader is located in one of the listed countries, you can still apply the protocol.
An example of a situation in which selling on fob conditions is allowed: a German feed producer purchases sunflower seed meal from Ukraine via a trader located in Belgium. In this case, the trader can sell the product on fob conditions because sunflower meal from Ukraine is an ‘allowed product’.
An example of a situation in which selling on fob conditions is NOT applicable: a German feed producer purchase soybean meal from Brazil via a trader located in The Netherlands. In this case, we are talking about a forbidden product, gatekeeping is not allowed. Protocol cannot be used at all.
Which options do I have when purchasing processed feed materials via gatekeeper protocol 4.3.8?
There are two options:
- For an indefinite period of time, but with monitoring of each batch. Monitoring must be carried out in accordance with annex 1 of gatekeeper protocol 4.3.8. The option means that you can continue to buy a feed material from a certain producer for years, provided you analyse each batch for the defined parameters. You should not reduce the frequency and the determined parameters must all be analysed every time.
- For a limited time (max 18 months), with monitoring based on a hazard analysis. This 18-month period is intended for the non-certified producer to set up his own assurance system and have it certified. This must be demonstrated by a contract that the producer has concluded with a certification body.
Additional to the general notification requirement, if you want to use option 2, you need to send more information.
- A producer's contract with the certification body
- A substantiated monitoring plan
GMP+ International checks whether the information sent meets the requirements of the protocol and reports its findings to you. Only then can you use this option. Correct application of the protocol is verified by the auditor.
I purchase a certified feed additive or feed materials via a non-certified intermediate independent sales office. How to notify to GMP+ International?
In some countries, GMP+ certified companies can only buy feed additives, produced under certification, via a single independent sales office. This independent sales office is most of the time not (yet) GMP+ certified. The feed additive is delivered in the producer's original packaging.
Because the purchase and invoicing are done via a non-GMP+ certified independent sales office, the GMP+ chain is interrupted.
For this situation the feed additive gatekeeper protocol can be used.
We ask you to fill in the 'Gatekeeper Protocol Notification Form' in the following way:
- Additional information: the name and address details of the sales office.
- Upload the GMP+ (or equivalent) certificate of the producer.
In case of sampling of unprocessed grains from the collect chain (4.3.1), can I take samples while the product is stored?
It is possible to sample a batch in storage. This batch must be kept separate at the storage location until it has been sampled, analysed and released. You can then deliver this batch directly to the final recipient.
Example sampling batch in storage:
Suppose you want to transport the batch by trucks from the storage location. Because the batch has already been sampled, analysed and released (GMP+ assured) at the storage location, you are not obliged to also analyse every 20th truck. This also applies if you deliver to multiple recipients.
Suppose you want to store several smaller deliveries together as one batch. Then you must keep this batch separate until it has been sampled, analysed and released. You can then use this batch for the production of GMP+ feed.
How does sampling of trucks needs to be done?
If you receive grains or oilseeds by truck from various (non-assured) origins, you must sample each truck. You must analyse every 20th sample, unless you can store the loads and regard them as 1 batch and sample them (see previous question).
How to define what pesticides should be analysed on in unprocessed grains?
This should be defined on the basis of a risk analysis. When it is certain that certain pesticides have not been or are not used during cultivation, you do not need to analyse them.
Can you explain the limitations for selling a former foodstuff to another company?
As GMP+, we prefer that company that purchases a former foodstuff which needs a treatment to become a feed, is able to carry out this treatment himself. Examples: unpacking, drying, cleaning. This company must have a validated process which is GMP+ certified (scope: Production of feed material).
However, in some cases a company cannot carry out this treatment. Then he can resell this former foodstuff to another company that is able to carry out this treatment (a producer). These limitations for reselling are only applicable for former foodstuffs that need a special treatment to become a feed.
These limitations are not applicable when the former foodstuff is to be considered as feed and can be fed to the animals or processed into a feed.
We noticed that the clause in the GMP+ TS 1.2, protocol 4.3.4 are read and interpreted in different ways.
What requirements should be followed when purchasing herbs and spices from a non-certified company?
- When production is done according to a GFSI recognized scheme, these herbs and spices may be considered as food and should be purchased under gatekeeper protocol 4.3.3 within TS 1.2. When production is not done according to a GFSI recognized scheme, these herbs and spices should be purchased under gatekeeper protocol 4.3.7 within TS 1.2.
- For Europe, the following herbs and spices are involved:
- Products from the European feed materials catalogue (Reg. (EU) No. 68/2013) listed in category 7.
- 7.3.1 Bark
- 7.4.1 Blossoms, dried
- 7.7.1 Leaves, dried
- 7.9.1 Liquorice
- 7.10.1 Mint
- Products not listed in category 4 or 7 of Part C of the European catalogue of feed materials, such as roots, rhizomes, tubers or cereals of a vegetable species. The feed material (carrot or grain) must be mentioned in the list published on the website www.feedmaterialsregister.eu.
- All relevant herbs and spices are subject to a generic risk assessment included in the FSP.
We use the gatekeeper protocol for a non-certified company for road transport / inland waterway transport / storage of GMP+ assured feed. Is that company allowed to outsource this activity with assured feed to another non-certified company?
No, this is not allowed. As the gatekeeper you can only take responsibility for service provided by the company you have a quality/ feed safety assurance agreement with and which is checked by you on the compliance with the agreement, GMP+ requirements and applicable feed legislation.



