1. General
1.1. Who can use GMP+ Monitoring database and where to find it?
All GMP+ certified companies can use the GMP+ Monitoring database without additional costs. The GMP+ Monitoring database can be found in the GMP+ Portal.
1.2. How do I sign up for the GMP+ Monitoring database?
If a GMP+ certified company has an activated account for login to the Portal, this account can be also used for GMP+ Monitoring database.
If the GMP+ certified company does not have an activated account for the Portal, it can send a request with full name and email address of the GMP+ contact person to the Helpdesk. The email address will receive an email with the instruction of setting an own password.
Please note that the email may end up in the spam box!
1.3. Why is the GMP+ Monitoring database available in no more than 3 languages?
The content of the GMP+ Monitoring database is subject to so many changes, that it would be too complicated to manage the monitoring database in other languages than Dutch, English and German. We thank you for your understanding.
2. Adding analysis result
2.1. Who can add analysis results?
Only the GMP+ certified companies and/or laboratories of the GMP+ certified companies can add the analysis results into the database. GMP+ International cannot do this for GMP+ certified companies.
2.2. What are the points of attention when adding analysis results?
- The moisture content of your product. Most of the feed safety limits in TS 1.5 are based on a moisture content of 12%. In case your product deviates from this 12% moisture content please calculate your result accordingly. During manual submission of analysis results a calculation tool is available.
- The correct unit of your indesirable substance. The unit for an undesirable substance is provided within the GMP+ Monitoring database. After selection of the undesirable substance, the corresponding unit will appear. In case of digital submission, default units can be found in the 'parameter code' list.
See question 2.6 if different units are used in the report and GMP+ Monitoring database.
- The correct symbol ('<' or '='). The '<' symbol means that, at least up until the reported value, the substance was not found in the sample (this is the detection limit of the analysis method). The '=' symbol means that a certain amount of the indesirable substance is actually found. So it is important to use the correct symbol because the interpretation of both is different.
2.3. How do I prevent the same analysis result from being entered into the GMP+ Monitoring database more than once?
Once you have logged in with your own username and password, you'll be notified when you try to resubmit the same result. This is to prevent results from being entered more than once.
It is possible to share the analysis results in the GMP+ Monitoring database, with your customer or supplier for instance. Instead of sending a copy of the analysis report via e-mail / fax / mail, you can also share the analysis result within the GMP+ Monitoring database. This allows you both to gain access to the analysis results entered into the system by one of you. This also prevents the result from being entered into the GMP+ Monitoring database several times.
2.4. How do I easily enter my pesticide analysis?
An analysis for pesticides is a fairly extensive analysis with many analyzed components. Entering them, can be a tough job, if it has to be done manually. However, there is a way to do it quickly and easily.
- When registering the sampling, don't select all individual pesticides, but select the indesirable substance “Crop protection agents / biocides”.
- Create an analysis set (once) (under personalize) and include all pesticides which are usually reported by your laboratory. These pesticides are part of your pesticide set. In this analysis set, you can also indicate the detection limit of the individual pesticides. When no pesticide is detected, it will be reported based on this detection limit. By documenting the detection limits once, the GMP+ Monitoring database can automatically insert them into the fields, so that you only have to enter the values of any pesticides found.
When something changes in the pesticide package (such as the detection limits or the pesticides included in the package), you can always change it at any time. - If you wish to enter the analysis report, select the analysis set you have created in that section of the page. Subsequently, the system will copy all pesticides from your analysis set to the analysis result, including the detection limits defined by you.
- Using the red x behind the line, remove the substance “Crop protection substances / biocides” from the analysis report.
Please note: If you are registering the sampling when you are also about to enter the analysis report, you can skip step 1 and 4.
Note 1: when you ask your laboratory to submit the analysis results via an Excel or XML file, they will report all individual pesticides (unless agreed otherwise with your laboratory). In that case, you don’t need to create an analysis set.
Note 2: this way, you don’t just submit the positive results but all results (including the pesticides measured, but not found). This allows for the creation of better report, since it allows you to see in how much of how much analysis, pesticide was found.
2.5. Can a laboratory submit analysis results via a digital message?
Indeed it is possible to submit analysis results in a digital manner. This is done by means of an electronic message (Excel or XML). You can choose to have a sample supplemented that has already been registered, or you can ask your laboratory to provide all information about the sample, including analysis result in an electronic message. Of course, the electronic message must meet certain requirements. Read more about the requirements for digital submission of analysis results in the manual of the GMP+ Monitoring database and on this webpage of the GMP+ website.
In case the laboratory experiences difficulties, they can contact the GMP+ Helpdesk.
2.6. The unit of my analysis results does not match the unit of the GMP+ Monitoring database, what should I do?
To be able to submit the result, you must enter the result with the same unit as requested in the GMP+ Monitoring database. The units in the GMP+ Monitoring database (where applicable) are in accordance with the standards that apply to the relevant undesirable substances. This allows the database to compare your results with the standard.
If the unit of your result deviates from the unit in the system there are two things you can do:
- Convert the analysis result so that it meets the unit. In the GMP+ Monitoring database you’ll find a ‘unit calculator’ under ‘related items’ which you can use to convert your analysis result.
- Although you can convert the analysis result to make the unit of the result match the unit in the database, it would be much easier to ask your laboratory to report in the unit requested by the database. This means that you would no longer have to convert the results and it would allow you to compare the analysis result with the standard more easily, without the database.
2.7. How do I submit a complete analysis result in as little steps as possible?
Although the GMP+ Monitoring database provides plenty of possibilities for various features, including adding additional information, you are not required to use these possibilities. In this paragraph, we will skip all additional options and describe how to submit your analysis results in as little steps as possible.
- In the main screen ‘My Monitoring’, click ‘enter sampling’.
- Only fill out the mandatory fields (product, sample number and sample date). If you have already received the analysis results, you can proceed to step 4. If you have not yet received the results and would only like to register a sampling, please proceed to step 3.
- Click save and back to overview to go back to the overview screen. As soon as you have received the analysis result, you can click the pencil in the overview screen to continue entering data.
- Click ‘add analysis report’.
- Only fill out the mandatory fields (undesirable substance, result (symbol and value)). Click the button ‘add’ behind each undesirable substance to save that line and to create a new line.
- Click ‘Save’ and then ‘Finalize’. Check with whom you would like to share a result (for instance anonymously with the GMP+ Community in the context of compliance with GMP+ requirements).
Please note; you can do this even faster by only following step 1, 2 and 6 yourself. The laboratory can add the analysis results to a registered sampling via an XML or Excel file.
3. Testing
3.1. Can I change a finalized sample?
No, as soon as you have finalized a sample, you can no longer change it. If you have not yet received all analysis results for instance, you can edit a sample by saving changes while you’re editing. Once you are ready, you can finalize the sample so that you can include it in your reports, and the sample can be shared. Of course, the consequence of finalizing is that changes are no longer possible. In other words, please thoroughly check your data before finalizing a sample.
If you found a mistake in your entry, please contact the GMP+ Helpdesk and together we can see (by exception) if there is any way to correct this.
3.2. Whenever I enter results into the database the analyze results colour green, orange or red. What does this mean?
The colour gives an indication whether or not the result of the analyze is above or below a certain limit. Within the GMP+ Monitoring database you will see an action limit and a rejection limit. The actual limits come from the TS1.5 Specific Feed Safety Limits. The limits within the GMP+ Monitoring database are the lowest action limit and the highest rejection limit that is known for the specific undesirable substance. There is no specific limit for every feed product that is included in the analysis result.
Below you will see an example of an undesirable substance and its limits. The yellow marked action limits and rejection limit are usually what can be found in the GMP+ Monitoring database.
| Contaminant | Products | Action limit | Rejection limit |
| Undesirable substance X | Feed materials of vegetable oils | 0,8 mg/kg | 1,2 mg/kg |
| Feed materials of animal origin | 1,6 mg/kg | 2 mg/kg | |
| Premixtures | 15 mg/kg | 18 mg/kg |
When there would be an analyze result entered into the GMP+ Monitoring database for substance X with a result higher than 18 mg/kg it will show in red.
When there would be an analyze result entered into the GMP+ Monitoring database for substance X with a result between 0,8 and 18 mg/kg it will show in orange.
When there would be an analyze result entered into the GMP+ Monitoring database for substance X with a result below 0,8 mg/kg it will show in green.
Whenever there is an analyze result entered from which no limit is specified in the TS 1.5 document, it will always show in orange.
3.3. What do I need to do when a result shows in red?
This means that one or more of the results in this sample exceeds highest rejection limit from TS 1.5. Within the TS 1.5 there could be limits per feed product or product group. Therefore it is required to assess the results by checking what limit is applicable for the specific feed product that has been ana-lysed. If there indeed is an exceedance of the highest rejection limit, the GMP+ requirements dictate how to deal with 'non-conforming products'. Don't forget to actively report this exceedance via the EWS procedure.
3.4. What do I need to do when a result shows in orange?
This means that one or more of the results in this sample exceeds the lowest action limit, but not the highest rejection limit from TS 1.5. Please check the result against the feed safety limits from TS 1.5 to be certain whether your product meets the limit that applies to your product.
If no limits are specified in TS 1.5, the result will fall in this category as well. In that case, you will have to assess whether this result meets the product limit as specified by you in your HACCP.
3.5. What do I need to do when a result shows in green?
This means the limit for this undesirable substance are not exceeded. It is not need-ed to take any further actions.
4. Personalization
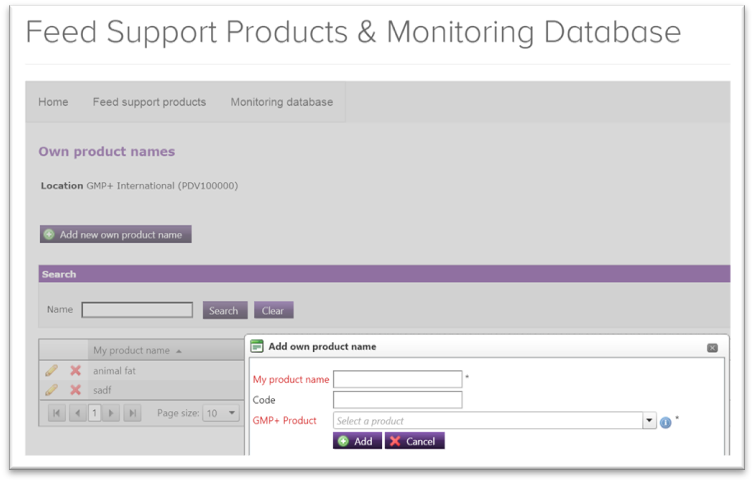
4.1. How can I include own product name in the GMP+ Monitoring database?
It is possible to include own product names in the database if the product belongs to a feed which is already included in the GMP+ Monitoring database. You can include own product name by doing as follow.
- Click on ‘Own product names’ in your monitoring database.
- Click on the icon

- Add own product name in ‘My product name’, and choose the type of feed your product belongs to in ‘GMP+ Product’.