1. General
1.1. What is GMP+ FRA certification?
FRA stands for Feed Responsibility Assurance. As part of the GMP+ Feed Certification scheme, the GMP+ FRA module contains requirements for the assurance of the production and / or trade of responsible feed. Via independent certification, the GMP+ certified company can demonstrate compliance with the requirements for producing and / or trading responsible feed.
GMP+ FRA certification is particularly interesting for companies with a GMP+ FSA (Feed Safety Assurance) certificate. The reason for this is that the system requirements for assuring responsible feed are very similar to the system requirements for assuring safe feed. This makes it very interesting to combine both certifications: a one-stop-shop multiple certification, allowing 1 auditor to include both aspects in his audit. This saves time and money. However, the certification can also be used in combination with another feed safety certification or as stand-alone.
1.2. What documents are part of the FRA module?
The GMP+ FRA module consists of two components:
- GMP+ FRA Framework
The GMP+ FRA Framework contains the system requirements for assuring responsible feed. These requirements have a lot of overlap with the GMP+ FSA standards, such as the procedures and registrations for tracking & tracing and for the selection and evaluation of suppliers.
In addition, general system requirements (management responsibility, staff matters, internal audit etc.) are also necessary for the continuous assurance of requirements for responsible feed, just as they are required for assuring feed safety requirements.
The GMP+ FRA Framework consists of the R5.0 Feed Responsibility Management Systems Requirements.
- GMP+ MI documents
The GMP+ MI documents contain the scopes and criteria for responsible feed. These MI documents have been established in consultation with the market initiative referred to on the cover sheet and the introduction. This market party has provided the definition of responsible feed and asked GMP+ International to provide independent certification for it.
Companies who wish to be certified for a scope from one of the MI documents, always do this in combination with R5.0. The GMP+ MI document states what parts from the R5.0 apply.
1.3. How does the combined use of R 5.0 and MI document work?
All MI documents have a clear reference to the R5.0 Feed Responsibility Management Systems Requirements. All mentioned parts of the R5.0 must be implemented to ensure compliance with the requirements in the MI documents. The auditor will check compliance with both documents during the audit.
1.4. How can I combine a GMP+ FRA certification with a GMP+ FSA certification?
Although GMP+ FRA certification is possible without additional (feed safety) certification, most feed companies will apply GMP+ FRA certification as an addition to certification for scopes of the GMP+ Feed Safety Assurance (FSA) module.
To facilitate this multiple certification, GMP+ International has integrated certification for both feed safety assurance and feed responsibility assurance in a single certification scheme (the GMP+ Feed Certification scheme). This prevents overlap of requirements, ensures uniformity in standards and conditions and allows for limiting the (administrative) burden of audits and certifications. One (successful) audit can result in certification of multiple scopes.
However, it is the responsibility of the feed company to identify the overlap between the GMP+ FSA module and R5.0 Feed Responsibility Management Systems Requirements, and to implement all relevant conditions into one management system which ensures both compliance with the feed safety standards and the requirements in the FRA module. Compliance will be verified during the audit.
1.5. Do I have to make a lot of adjustments to my system to be certified for the GMP+ FRA module?
Although there are a lot of requirements – in particular in the R5.0, it is relatively easy to implement the GMP+ FRA requirements. In fact, the only thing it requires is to view your current Feed Safety Management System through different eyes. This means, for instance, that your manual must include a procedure for informing your customers about the status of the feed.
All requirements listed in chapter 4 of the R5.0 match requirements from the GMP+ FSA standards. For that reason, Annex 1 of the document contains a cross-reference table to identify the origin of these requirements.
New in the GMP+ FRA module (in relation to the GMP+ FSA certification) is the Material Accounting System. This is an extensive version of a tracking & tracing system, in which you administratively document how much responsible feed you receive and how much you sell. This must be in balance.
2. MI documents
2.1. Which MI document applies to me?
GMP+ FRA certification is not required, but may be asked by your customer. Therefore, which MI document applies to you is largely determined by what the customer asks of you. Regardless of that, below you will find a brief overview of the various types of companies with associated (possible) MI documents. All this is in line with the following schematic representation in which a bridge is made between ‘chain of custody certification’ and the delivery to subsequent links:
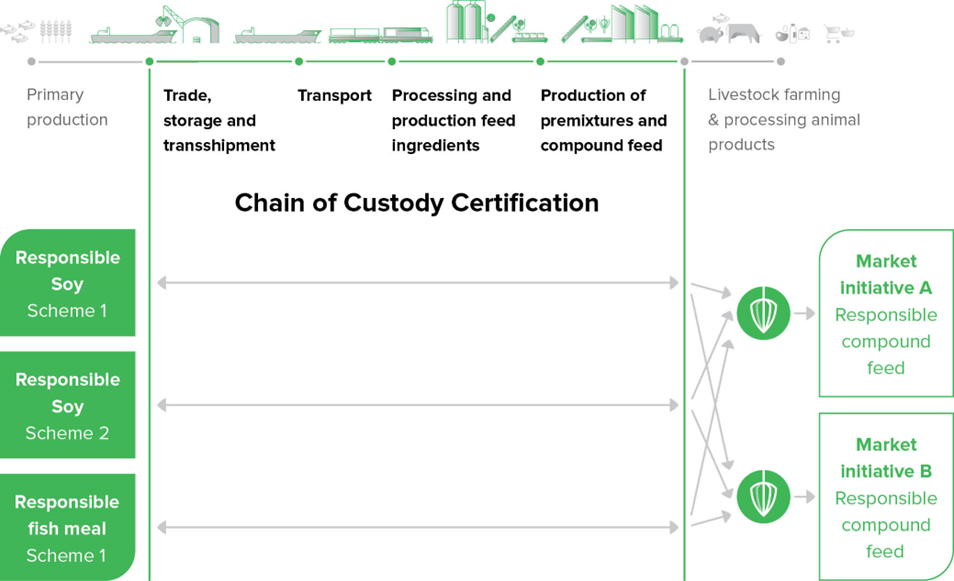
In the ‘chain of custody’ (supply chain) it is unknown what an individual raw material may be used for in the end. Therefore, a certificate for sustainable cultivation is aimed at for the chain of custody. In the chain of custody, it is assured that this sustainably grown raw material passes through the chain in a correct manner.
Only at the time of delivery of a feed material or a compound feed to the farmer, the connection to a market initiative can be made. This means that the turning point is at the direct delivery to the farmer.
2.2. What is the role of GMP+ International in determining the requirements in the MI documents?
GMP+ International works with a plug-in model for the assurance of responsible feed. GMP+ International offers a so-called GMP+ FRA Framework, providing the basis for assuring responsible feed. This GMP+ FRA Framework consists of a Feed Responsibility Management System and certification requirements. Subsequently, various market initiatives can be plugged into this GMP+ FRA Framework.
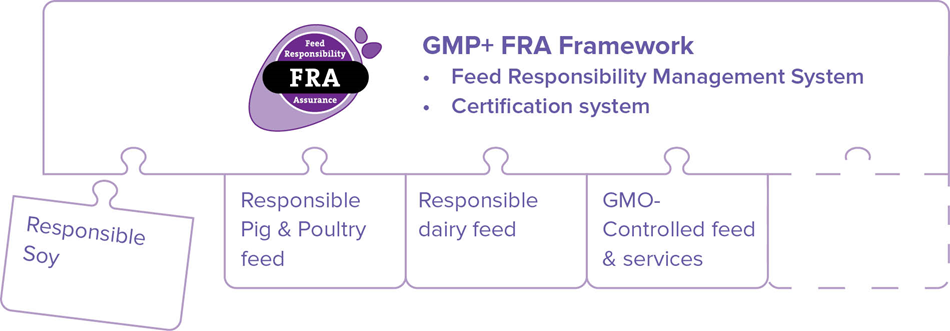
GMP+ International helps formulate the requirements defined by the market initiative for responsible feed. The reason for this is that these requirements must be concrete enough to be applied and to be auditable by an auditor. However, the market initiative is the party that determines what responsible feed is and what is included in the MI document. The subcommittee Responsible Feed tests the MI document against a number of requirements, after which it can be included in the GMP+ FRA module.
The requirements for including a MI document in the GMP+ FRA module can be found in the ”Feed Responsibility Assurance Policy”.
3. MI 5.1 Production and Trade of RTRS Soy
3.1. What is the scope of the MI5.1 document?
The MI 5.1 document contains the requirements to produce and/or trade RTRS certified soy in the supply chain. RTRS certified soy can be produced and/or traded in accordance with the supply chain models Mass Balance and Segregation.
3.2. What does RTRS consider as responsible feed?
RTRS soy does not only meet the highest environmental criteria (including a guarantee of third party-verified zero deforestation and zero conversion) but also a wide-reaching set of social and labour requirements.
RTRS certification is based on five principles:
- Legal Compliance and Good Business Practices
- Responsible Labour Conditions
- Responsible Community Relations
- Environmental Responsibility
- Good Agricultural Practices
3.3. Who will ask for feed that is in compliance with MI 5.1?
Feed producers that want to purchase RTRS Segregated soy or RTRS Mass Balance soy will look for a supplier that is certified for the applicable scope. The feed producer can choose to buy from a RTRS Chain of Custody certified supplier or a (GMP+) MI 5.1 certified supplier.
3.4. How does MI 5.1 relate to RTRS certification?
The certification scheme of RTRS offers two certifications:
- RTRS Standard for Responsible Soy Production (intended for the primary production of RTRS soy)
- RTRS Chain of Custody Standard (for the subsequent links in the chain)
The scopes Mass Balance and Segregation within the MI 5.1 Production and Trade of RTRS Soyare equivalent to the scopes Mass Balance and Segregation within the RTRS Chain of Custody Standard. This is also confirmed in an agreement between RTRS and GMP+ International.
Because of this, participants in the MI 5.1 are permitted to use the RTRS logo and to supply to RTRS participants.
4. MI 5.2 Responsible Pig & Poultry feed
4.1. What is the scope of the MI5.2 document?
The MI5.2 document contains:
- the requirements to produce and/or trade responsible soy and
- the requirements to produce and/or trade responsible compound feed.
The responsible feed is intended for pigs and/or poultry.
4.2. What does SMK consider as responsible feed?
All soy (including soybeans, soy products and soy derivatives) in responsible pig & poultry feed, must be responsible soy. Responsible soy is defined by SMK as soy produced and traded according to the requirements in the MI5.2 document.
The MI5.2 document recognizes several standards for the primary production of soybeans and for the production and trade of feed. Recognized are standards for primary production of soybeans that are benchmarked to the requirements in the following order:
- FEFAC Soy Sourcing Guidelines 2023
- Inspection system based on the EU Best Practice Guidelines for voluntary certification schemes for agricultural products and foodstuffs
- Cut-off date no later than May 2009
For the purchase of the responsible soy SMK allows the following 5 supply chain models:
- Identity Preserved
- Segregation
- Mass Balance
- Area Mass Balance
- Book & Claim
4.3. Who will ask for feed that is in compliance with MI 5.2?
SMK includes in the purchasing requirements of the Certification Scheme ‘On the way to PlanetProof’ for animal products for eggs (laying hens) that laying hen farmers must purchase their feed from companies that are certified for the MI5.2. These laying hen farmers will ask for this.
Of course it is possible that other Market Initiatives or pig and/or poultry farmers (that are not participating in the Milieukeur certification) require certification for the MI5.2.
4.4. What feed can be certified via MI 5.2?
MI5.2 is suitable for the production and trade of responsible soy and responsible compound feed intended for pigs and poultry. Since the requirements relate to the production and trade of responsible soy and responsible compound feed, the certification only relates to the part of the feed that contains responsible soy.
4.5. What is the validity of credits?
The MI5.2 document follows the requirements from the recognized standards stated in the MI5.2 document concerning the validity of the credits.
The validity of credits depends on the soy standard.
4.6. Is redeeming of credits part of the purchase?
Yes, where the MI5.2 document refers to the purchasing of credits, this includes the step ‘redeeming’ that is part of the purchasing process.
5. MI 5.3 Responsible dairy feed
5.1. What is the scope of the MI 5.3 document?
The MI5.3 document contains:
- the requirements to produce and/or trade responsible soy and
- the requirements to produce and/or trade responsible compound feed.
The responsible feed is intended for dairy.
5.2. What does the Sustainable Dairy Chain consider as responsible feed?
All soy (including soybeans, soy products and soy derivatives) in responsible dairy feed, must be responsible soy. Responsible soy is defined by the Sustainable Dairy Chain as RTRS soy.
For the purchase of the responsible soy the Sustainable Dairy Chain allows the following 3 supply chain models:
- Segregation
- Mass Balance
- Book & Claim
5.3. Who will ask for feed that is in compliance with MI 5.3?
The Sustainable Dairy Chain requires its members to include in their purchasing requirements that dairy farmers must purchase their feed from companies that are certified for the MI5.3 standard. These dairy farmers will ask for this.
The Sustainable Dairy Chain has indicated that the demand for MI5.3 certified feed applies to all feed delivered to the dairy farmer (not just for the lactating cows). The dairy farmer will concretely define for which feed he demands this certification.
Of course it is possible that other Market Initiatives or dairy farmers (that are not participating in the Sustainable Dairy Chain) require certification for the MI5.3.
5.4. What feed can be certified via MI 5.3?
MI5.3 is suitable for the production and trade of responsible soy and responsible compound feed intended for dairy.
Since the requirements relate to the production and trade of responsible soy and responsible compound feed, the certification only relates to the part of the feed that contains responsible soy.
5.5. What is the validity of RTRS credits?
The GMP+ MI5.3 document follows the requirements from RTRS concerning the validity of the RTRS credits.
From the moment the first company purchases the RTRS credits from the primary producer of soy these RTRS credits will be valid for 2 calendar years. The RTRS credits expire at the end of the year following their purchase.
5.6. Is redeeming of RTRS credits part of the purchase?
Yes, where the GMP+ MI5.3 document refers to the purchasing of RTRS credits, this includes the step ‘redeeming’ that is part of the RTRS purchasing process.
6. MI 5.4 GMO controlled
6.1. What is the scope of the MI 5.4 document?
The scope of the standard is defined as ‘GMO controlled’ and can be used for:
- The production of GMO controlled compound feed, feed materials, feed additives and premixtures
- The trade of GMO controlled compound feed and/or feed materials
- The storage and transshipment of GMO controlled compound feed and/or feed materials
- The transport of GMO controlled compound feed and/or feed materials
6.2. What does VLOG consider as responsible feed?
The VLOG Standard is based on the GMO labelling provisions of Regulations (EC) 1829/2003 and 1830/2003. Contamination with GMOs permitted in the EU by law does not require labelling according to Regulations (EC) No. 1829/2003 and No. 1830/2003 provided that two requirements are fulfilled:
- The threshold value of the GMO content of 0.9% per feed material is not exceeded and
- The presence of the GMO content is “adventitious or technically unavoidable”.
Certification for VLOG or the (GMP+) MI5.4 shows compliance with these regulations.
6.3. Who will ask for feed that is in compliance with MI 5.4?
Livestock farmers who deliver non-GMO food products (meat / milk / eggs) to the market, will ask their feed suppliers to deliver non-GMO feed. For these livestock farmers, it is required to feed their livestock with non-GMO feed in order to sell their food products such as dairy, eggs and meat as non-GMO products.
6.4. Does all feed needs to be covered under MI 5.4 certification?
No, only the feed of which the GMP+ certified company wants to make the claim that the feed is GMO controlled. MI5.4 can be applied for all compound feed, feed materials, feed additives and premixtures. All feed that are included in the scope of certification, must comply to the requirements in the MI5.4.
6.5. Do I need to make a Risk Assessment for other products than feed materials?
No, this requirement is only applicable for feed materials. Compound feed, feed additives and premixtures are excluded for this requirement.
In case a compound feed producer uses feed materials to produce the compound feed, the result of the risk assessment of the individual feed materials is used to manage risks for the compound feed.
As with GMP+ FSA certification, the requirements in the MI5.4 do not apply for products other than feed. If a trader sells (for example) straw as bedding to livestock farmers, this is not covered with the certification.
6.6. Why does not GMP+ International or VLOG publish a list of at risk / not at risk feed materials?
It is not possible for GMP+ International to make a generic risk assessment for all feed materials that are suitable for all situations.
VLOG sets the requirement to make a risk assessment to define at risk / not at risk feed materials (that GMP+ International follows in MI5.4), but also does not provide a list of at risk feed materials to its participants. VLOG does provide some guidance in following document:
An “Assessment Aid – At Risk Feed” is available on the VLOG homepage (under ‘Further Documents / Instructions’) to assist the feed business. This document includes a table which provides an overview of where growing genetically modified plants is allowed and thus possible at risk feed origin.
GMP+ International advises the participant to use this document (among all available information) to create its own risk assessment.
6.7. Do I have to sample and test incoming compound feed, feed materials, feed additives and/or premixtures which I have classified as ‘not at risk’?
No, this is not required as part of the GMP+ (and VLOG) certification.
However, for certain feed materials classified as ‘not at risk’ sampling and testing is required.
This concerns trading companies that purchase ‘not at risk’ soy, rapeseed, canola, corn/maize, sugar beet or cotton from a non-certified supplier and deliver it directly to the customer as GMO controlled. Every year at least 1 sample and 1 test are done.
6.8. Can I purchase from non-certified suppliers?
Yes, as long as the participant has a confirmation from the supplier of the GMO controlled status of the purchased feed.
6.9. What to do when my company currently has a VLOG certificate for GMO controlled feed?
When a company is currently certified for the VLOG standard it is possible to change to the FRA standard MI5.4. This can be arranged with the Certification Body which provided the VLOG certificate and has got acceptation for MI5.4 GMO controlled. Please contact your Certification Body for more information about the transition.
6.10. Is multisite certification possible for MI 5.4?
Yes, it is possible to get a multisite certification. The requirements are described in CR2.0 Assessment and Certification. Your Certification Body can give more information about this possibility.
6.11. Feed materials in which GMOs cannot be tested through a PCR test may not be purchased from non-certified suppliers and sold as GMO controlled. When is it not possible to detect GMOs in feed materials with a PCR test?
It is not possible to detect GMOs in a feed material with a PCR test if:
- the feed material has a lack of sufficient DNA (genetic material). According to the VLOG document “Suitability of GMO Analysis for Feed, Raw Materials and Food” this is the case for feed materials which are strongly processed, like soy bean oil, rape seed oil and glycerin.
- the feed material does not have sufficient detectable DNA anymore. This concerns feed materials, which in principle can be tested with a PCR test, but due to process steps, show fluctuations in the DNA amount. According to the VLOG document “ Suitability of GMO Analysis for Feed, Raw Materials and Food” this concerns for example soy lecithin, sugar beet (pressed) pulp.
The VLOG document “ Suitability of GMO Analysis for Feed, Raw Materials and Food” is available on the VLOG website (under ‘Further Documents / Instructions).
6.12. According to MI 5.4 I have to inform my customer about the status of the feed. Can I use the terms ‘GMO free’ or non-GMO’?
No, the claims ‘GMO free’ and ‘non-GMO’ suggest the absence of GMOs. It is technically impossible to say that there is no GMO present in a sample. Therefore the term ‘GMO controlled’ declares that the feed is produced, traded, stored or transported in compliance with the requirements of the MI5.4.
6.13. As a GMP+ certified company I want to label my products with the VLOG geprüft seal. Am I allowed to do this?
Yes, you are allowed. More information on the use of the VLOG geprüft seal can be found via the VLOG website:
https://www.ohnegentechnik.org/fuer-unternehmen/vlog-geprueft-siegel-futtermittel/vorteile
6.14. Is it allowed to use the statement ‘GMO controlled’ in Belgium?
Yes. We’ve received questions whether is allowed to use the statement ‘GMO controlled’ in Belgium (because it is thought to be conflicting with legislation). The following confirmation from the Belgium authority FAVV shows that there is no legal objection to use the GMO controlled statement in Belgium:
“I have contacted our local control authorities and according to the information in my possession, I have not received any feedback confirming that inspectors are of the opinion that GMP+ certified feed companies in Belgium cannot use the declaration “GMO Controlled”.
The FAVV controls claims on animal feed in accordance with article 13 of Regulation (EC) No 767/2009. In the context of GMP+ standard MI5.4 GMO Controlled, the "GMO controlled" claim is objective and verifiable.
We have forwarded this information to our local control authorities. “
7. MI 5.5 Carbon footprint of feed
7.1. What is the scope of the MI 5.5 document?
The MI5.5 document contains the requirements for the calculation of the carbon footprint (CFP) of feed and its communication towards customers or third parties.
The MI5.5 document ensures the calculation of the CFP of feed in accordance with the methodology of the Nevedi Protocol.
The MI5.5 document is applicable to the compound feed company, located in the Netherlands, producing and delivering compound feed to the buyer.
7.2. What is considered as responsible feed?
Feed of which the CFP is calculated in accordance with the methodology of the Nevedi Protocol.
7.3. Who will ask for feed that is in compliance with MI 5.5?
Dairy farmers who want information about the CFP of feed delivered to the central database ‘Kringloopwijzer’.
Of course it is possible that other livestock farmers will ask for feed certified when they are interested in knowing the CFP of the feed.
7.4. What feed can be certified via MI 5.5?
Currently, MI5.5 can only be applied for compound feed.
8. MI 5.6 Production and Trade of responsible feed
8.1. What is the scope of the MI 5.6 document?
The MI5.6 document contains:
- the requirements to produce and/or trade responsible feed materials and
- the requirements to produce and/or trade responsible compound feed.
The responsible feed is intended for food-producing animals and/or pet animals.
8.2. What is considered as responsible feed?
All soy (including soybeans, soy products and soy derivatives) in responsible feed, must be responsible soy. Responsible soy is defined as soy produced and traded according to the requirements in the MI5.6 document.
The MI5.6 document recognizes several standards for the primary production of soybeans and for the production and trade of feed. Recognized are standards for primary production of soybeans that are benchmarked to the requirements in the following order:
- FEFAC Soy Sourcing Guidelines 2023
- Inspection system based on the EU Best Practice Guidelines for voluntary certification schemes for agricultural products and foodstuffs.
For the purchase of the responsible soy it is allowed to use the following 3 supply chain models:
- Segregation
- Mass Balance
- Book & Claim
8.3. Who will ask for feed that is in compliance with MI 5.6?
QS and QM Milch require companies delivering soy and compound feed containing soy to their chain to implement requirements of a recognized responsible soy standard as of 01-01-2024. Certification for a recognized responsible soy standard is required no later than 31-12-2024.
GMP+ certified companies certified for the MI5.6 can continue delivering soy and/or compound feed containing soy to the QS and/or QM Milch chain. The MI5.6 standard meets the requirements of QS and QM Milch, and is recognized.
Of course it is possible that other Market Initiatives and/or companies (that are not delivering in the QS and/or QM Milch chain) require certification for the MI5.6.
8.4. What feed can be certified via MI 5.6?
MI5.6 is suitable for the production and trade of responsible soy and responsible compound feed intended for food-producing animals and pet animals. Since the requirements relate to the production and trade of responsible soy and responsible compound feed, the certification only relates to the part of the feed that contains responsible soy.
8.5. What is the validity of credits?
The MI5.6 document follows the requirements from the recognized standards stated in the MI5.6 document concerning the validity of the credits.
The validity of credits depends on the soy standard.
8.6. Is redeeming of credits part of the purchase?
Yes, where the MI5.6 document refers to the purchasing of credits, this includes the step ‘redeeming’ that is part of the purchasing process.
Appendix: Cross-reference
Cross-reference between the System Requirements of R5.0 Feed Responsibility Management Systems Requirements and the relevant GMP+ FSA standards.
| R 5.0 Feed Responsibility Management Systems Requirements | TS document | ||||||
| 4.1 Feed Responsibility Management System | | | |||||
| 4.1.1 Leadership and commitment | 5.1 | | |||||
| 4.1.2 Top management’s responsibilities and authorities | 5.3.1 | | |||||
| 4.1.3 Determining the scope of the Feed Responsibility Management System | 4.3 | | |||||
| 4.1.4 Documented information | 7.5.1 | 7.5.2 | 7.5.3 | | |||
| 4.2 Prerequisite programmes (PRPs) | | | |||||
| 4.2.1 People | 7.1.2 | 7.2 | 7.3 | | |||
| 4.2.2 Traceability system | 8.3 | TS 1.1, § 10 | |||||
| 4.3 Risk Assessment | 8.5.2.2 | | |||||
| 4.4 Purchase | | | |||||
| 4.4.1 Selection of suppliers | 7.1.5 | | |||||
| 4.4.2 Verification of incoming products and/or services | | TS 1.1, § 9.1 | |||||
| 4.5 Informing the customer | | | |||||
| 4.5.1 Inform the customer about the status of the feed | 7.4.2 | | |||||
| 4.5.2 Delivery requirements | 7.4.2 | | |||||
| 4.6 Verification | | | |||||
| 4.6.1 External communication | 7.4.2 | | |||||
| 4.6.2 Internal audit | 9.2 | | |||||
| 4.6.3 Management review | 9.3.1 | 9.3.2 | | ||||



